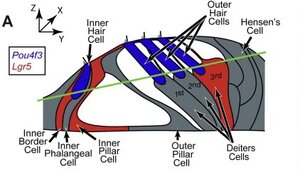
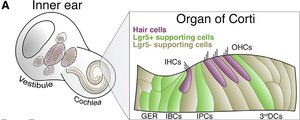
Despite all the well meaning public statements, Frequency Therapeutics is a for-profit company with stockholders. As a business, it doesn't make much sense to use that $42m fund on expanded access. It's better for them to quickly go through the whole clinical trials shebang for quick product release. Frequency Therapeutics still need the capital to establish phase 3, then setup larger production, marketing, etc. Despite many people having hearing loss and tinnitus, it's still not well known to the majority.
Also, Frequency Therapeutics have other drugs in development, which was mentioned in their press release, regarding the $42m fund, about putting effort into multiple sclerosis.
Also, I'm not trying to be negative, but getting our hopes up too high right now is premature. They're still in phase 2. Everything hinges on phase 2 results.

 Member
Member