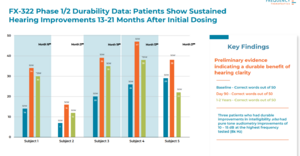
According to ClinicalTrials.gov, audiograms are being measured in the new Phase 2 trial, they just aren't being used as a primary outcome.
Imagine having to build a plane while you're flying it. That is what the company is doing right now as hearing regeneration has never been done before. The initial expected outcome of the audiology community was that there were going to be improvements in audiograms when regenerating extended high frequency cochlear hair cells but instead we are seeing improvements in speech perception first. Side note, audiograms are no more indisputable than word recognition tests. Its just as easy to pretend you don't hear a beep as it is to pretend you don't understand a word.
I know many around here consider audiograms to be the divine trinity or the holy grail of hearing measurement but as hearing regeneration science advances, we are learning more and more about the intricacies of the inner ear and hearing. Now we know that the health of the cochlea can determine both loudness of sounds & clarity of sounds and there is a lot of time being spent now on learning how the two are related and what anatomic parts of the cochlea are responsible for each.
For those so focused on audiograms and who have little interest in clarity, I pose this question to you. If you have a blown out speaker in your car or your surround sound system in your house and that speaker sounds absolutely terrible, scratchy & distorted, etc; would you be satisfied by just cranking up the speaker and listening to the distorted music louder? Would that improve the quality of the experience you get from that speaker? Or would you prefer to fix the distortion? Say you do manage to fix the distortion from that speaker; is that not considered an improvement to the sound coming from that speaker much like improving speech perception is an improvement to hearing?

 Member
Member