@d'Wooluf, this is what I was alluding to. I see a minuscule signal to show it does 'something' positive. I can accept that. However, like you said, even if it gets approved, it's got to be sellable. In Britain the NHS approve and buy in treatments based on efficacy. Is it cost effective? They would not buy this on the results seen so far.
Lenire is approved as a medical device in Europe but the NHS has not bought it for clinics because its effect is non-existent to minuscule for most people.
How can Frequency Therapeutics sell it to health boards based on the results we have seen so far?
The average punter doesn't think like us. They have hearing loss on an audiogram and want to see an improvement or have a treatment that makes them hear better on a day to day basis, not a speech-in-noise test. They would need to experience a significant improvement before having needles stuck in their eardrums...
You're losing the forest for the trees and you're thinking like a tinnitus sufferer rather than a hearing loss sufferer. You have to look at the totality of all the data to see where the value in FX-322 lies.
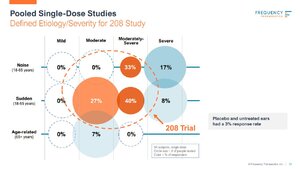
To start, FREQ was told by the FDA that they would need to conduct a number of probing studies into all the different etiologies & severities of hearing loss if they wanted to apply for the broadest indication possible for FX-322 and that's what FREQ did. Now that FREQ has 5 studies under their belt, they know who FX-322 is most effective on and where they have seen the greatest response which is in the moderately-severe population where they have seen up to a 40% response rate and this is the population they are loading the Phase 2b trial up with right now. It was their first 2 studies in which they saw a consistent response in similar etiologies & severities but since then, they have ran an additional 3 more studies in other populations that have not shown a robust response so many have written them off entirely, completely forgetting about their early results. Another thing to remember is it's not going to work for everybody due to heterogeneity of hearing loss combined with the fact that FX-322 is barely touching the tip of the cochlea as you can see on
slide 7 of their current investor slide deck.
You must also understand the test FREQ is using to determine statistically significant responders. They are using a 50 word recognition test modeled on Thorton & Raffin which defines statistically significant responders as improving by 10 words or more out of 50. It is generally agreed upon in the industry that a score of 20 words or less makes someone a candidate for a cochlear implant surgery which costs approx $30,000 to $50,000 before insurance. I've attached a graph showing the improvements subjects saw in their Phase 1/2 trial. Before receiving FX-322, both subject 1 and subject 3 qualified for a cochlear implant but after FX-322 administration, they no longer need a $30,000 surgery and can get by with hearing aids which only cost a couple thousand dollars. Subject 4 went from needing hearing aids to potentially being able to get by without them. I can guarantee an insurance company will be happy to shell out a few thousand dollars to pay for FX-322 for the chance that they don't have to shell out $50,000+ for a cochlear implant surgery.
The average hearing loss patient doesn't walk into their audiologist saying "I think I've lost 15 dB in my 4000 Hz range", most people go to their audiologist saying "I can't hear or understand the people around me". There are 2 parts of hearing; loudness and clarity. You say people only care about audiogram improvements so the best analogy I can think of to put it in perspective is, let's pretend you have a quiet, blown out speaker and you bring it in to get repaired and you tell them you want it louder but don't really care all that much about clarity. So the technician fixes your speaker and now you can crank it super loud but it still sounds blown out and garbled at a loud volume, are you going to be happy and satisfied with that?
I find it odd that you don't consider a speech-in-noise test improvement validation that someone can hear better on a day-to-day basis. There are people with hearing loss who use hearing aids that are still unable to go out to a loud crowded restaurant because they can't hear the people around them because they can't understand speech-in-noise. Even though some may think the results from FX-322 are disappointing, I can guarantee you it is life changing results for those that have been statistically significant responders.
To circle back to FX-322's lack of cochlear penetration, the fact we are seeing at least some responders is validation that FREQ is targeting the right pathway in the ear and bodes very well for FX-345 given its predicted cochlear penetration.
The last thing to consider is that current audiologic exams may not be capturing all the improvements patients are seeing. I recall a couple of years ago that someone shared that they were in one of the FX-322 trials and they said that they could tell the direction that sound was coming from better after participating in the trial and that is a perfect example of something that can't be captured with current exams right now. That is the reason the company has invented RADIAL, a patient report outcome which is a 40+ question survey asking patients how their hearing affects their daily life and is another way they may be able to capture improvements in order to get reimbursement from insurance companies.
If you don't see the value in a compound that can prevent people from going through an invasive, $30,000+ surgery, then there isn't much more I can say to change your mind.

 Member
Member