Yep. It's only meaningful when you can work out what's changed.So maybe it's because they only did the pre FX-322 audiograms up to 8kHz.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Frequency Therapeutics — Hearing Loss Regeneration
- Thread starter RB2014
- Start date
More options
Who Replied?- Feb 19, 2020
- 65
- Tinnitus Since
- 11/2017
- Cause of Tinnitus
- Jet Engines, Concert
Why regenerating functional hair cells looks so promising:
https://www.sciencedirect.com/science/article/pii/S0929664615003848
https://www.sciencedirect.com/science/article/pii/S0929664615003848
Attachments
True, you don't get high frequency tinnitus normally through aging, and that wasn't my point.Well I don't see why they wouldn't be able too. Regardless, just because you age does not mean you will develop high frequency tinnitus.
My point is that through aging, we naturally lose hearing, which if we age past the tinnitus tone frequency, say 16kHz, and we're now 40 and can hear 15kHz but not 16kHz anymore, have we gotten so profoundly deaf on that range that you have no more support cells? If they're able to restore all upper frequency ranges, then this isn't an issue. It means that the support cells needed for effectiveness of FX-322 is well within what you can expect to remain in the human ear, even if you can't hear it.
If not, then it means there's a window on the usefulness. So, if this really helps tinnitus, then it would be really useful soon after the inciting incident, as the progenitor cells would be freshly exposed, and then could be regenerated even if it was traumatic loss. This is my worry with all regenerative treatment, even synapse regeneration. If there's no living hair cell on the high frequency range of tinnitus for the synapse to connect to, how will it help?
Then again, I could be wrong. I just think that if regenerating hair cells is the plan, and you need progenitor cells to do it, and we have natural hearing loss in the high frequency ranges, and at a certain point with enough hearing loss you have flat epithelial cells and not progenitor cells, then you have a time limit on treatment. This might not be the case though, restoring other high frequencies might cause the brain to wake up and ignore the loss? Who knows? At this point, it's all speculation, and the only ones that know the answer are those in the trial, but as it's double blind they're not even fully certain.
Maybe I'm just overly pessimistic, but a lot of the testing they've done to see the effectiveness is to kill hair cells in the cochlea and then apply the drug immediately after. So I'm concerned on its abilities for chronic damage beyond a certain time frame. As I stated, I hope my concerns are completely unwarranted. I hope it restores it all, and if there remains synapse issues that OTO-413 or the Hough Pill can fix them, with there being living hair cells for the synapses to connect to because of FX-322. However, if not, my belief would lay in Xenon's Kv7.2 and 7.3 modulators in the pipeline, as well as RL-81. Susan shores device is interesting, but it too would have the issue of hearing loss by aging.
I finally had a physical appointment with my ENT today, it barely lasted 3 minutes. My hearing test was fine, so suck it up.It's a shame because a lot of tinnitus resides in upper frequencies. According to a standard audiogram, I have perfect hearing. The model is so outdated. All frequencies matter.
At least I managed to obtain a referral to the audiologist—after repeatedly pressing the matter.
I'm curious to find out what'll show up in an extended audiogram. Plus, hopefully the audiologist might be better able assuage my anxiety.
Edit: the ENT didn't even physically check my ears. Her assistant only gave me a hearing test prior to the main appointment. Is this normal? :/
I wasn't aware it was hard to get UHF in the States.It's not the time involved.
At least in the US where this study is taking place, it's highly unusual for clinics to have this ability. I live in a reasonably large area and I had to go across many states to get a high frequency audiogram done. Not because of the time involved but because the equipment wasn't calibrated for the UHF testing.
Edit: for anyone looking to get UHF testing done themselves, if I had known to go into a university setting, I could have gotten this done earlier. My mistake was trying to get UHF through regular ENT clinics.
UHF testing is routinely done for monitoring premature hearing loss in people on certain types of chemo. So if one would go to university hospitals you'd have a good chance of getting a UHF done, but you've found a way to do it anyways.
I'm just sort of baffled by the fact that they would have far more solid evidence of regeneration, and thus far more investor confidence and proof of effectiveness if they had only thought of UHF testing in their clinical trial. It was to be expected that the medicine would have to pass the apex of the cochlea first. Now they have a rather vague 10dB improvement at 8kHz which is fairly close to the margin of error of 5dB. Were they so bold to believe the results would be more favorable? Did they go "oops" after the trials were done?
Now we have to wait for phase 2 completion to get our answers.
- Feb 14, 2020
- 1,630
- Tinnitus Since
- 1-2019
- Cause of Tinnitus
- 20+ Years of Live Music, Motorcycles, and Power Tools
The objective of the Phase 1/2 was to validate a safety assessment. The standard measures for hearing (words in noise, clarity, audiogram) were not even technically part of the official clinical trial. So, they probably had to work with what was available at the single testing site in San Antonio. They also encountered a ceiling effect having people with mild hearing loss.I wasn't aware it was hard to get UHF in the States.
UHF testing is routinely done for monitoring premature hearing loss in people on certain types of chemo. So if one would go to university hospitals you'd have a good chance of getting a UHF done, but you've found a way to do it anyways.
I'm just sort of baffled by the fact that they would have far more solid evidence of regeneration, and thus far more investor confidence and proof of effectiveness if they had only thought of UHF testing in their clinical trial. It was to be expected that the medicine would have to pass the apex of the cochlea first. Now they have a rather vague 10dB improvement at 8kHz which is fairly close to the margin of error of 5dB. Were they so bold to believe the results would be more favorable? Did they go "oops" after the trials were done?
Now we have to wait for phase 2 completion to get our answers.
Most Phase 1 or 1/2 don't provide anything close to the meaningful data gained from a handful of participants.
I don't see this as much of an "oops" as a refining of their strategy to validate the effectiveness of the drug within the scope of generally accepted clinical methods used today.
I think they were so bold. They believed that they would do more on the lower ranges, as that's what they were really targeting. To be clear, they're trying to cure hearing loss, and that's mostly a problem on the 20 Hz - 8 kHz range. I imagine they were surprised they got as much improvement as they did for as little regeneration on the targeted ranges as they did. Honestly, I see this as a potential huge wake up call to the audio world how important these upper ranges are to hearing. We might actually see more UHF screening in the future if that happens.I wasn't aware it was hard to get UHF in the States.
UHF testing is routinely done for monitoring premature hearing loss in people on certain types of chemo. So if one would go to university hospitals you'd have a good chance of getting a UHF done, but you've found a way to do it anyways.
I'm just sort of baffled by the fact that they would have far more solid evidence of regeneration, and thus far more investor confidence and proof of effectiveness if they had only thought of UHF testing in their clinical trial. It was to be expected that the medicine would have to pass the apex of the cochlea first. Now they have a rather vague 10dB improvement at 8kHz which is fairly close to the margin of error of 5dB. Were they so bold to believe the results would be more favorable? Did they go "oops" after the trials were done?
Now we have to wait for phase 2 completion to get our answers.
Yes, in the light of them wanting to develop a cure for hearing loss it makes sense to want to regenerate the speech frequencies. There are much more people out there to market the product to.I think they were so bold. They believed that they would do more on the lower ranges, as that's what they were really targeting. To be clear, they're trying to cure hearing loss, and that's mostly a problem on the 20 Hz - 8 kHz range. I imagine they were surprised they got as much improvement as they did for as little regeneration on the targeted ranges as they did. Honestly, I see this as a potential huge wake up call to the audio world how important these upper ranges are to hearing. We might actually see more UHF screening in the future if that happens.
In comparison, tinnitus is less lucrative (but still very much so I believe).
If hearing regeneration works, tinnitus sufferers will likely benefit.
But if the hearing regeneration is unable to provide meaningful changes to speech perception and hearing, it may still do something for tinnitus, especially of the high pitched sort.
It is as if they made tinnitus their way out, as a secondary goal, in case all else fails.
Hi Bartoli,I wasn't aware it was hard to get UHF in the States.
UHF testing is routinely done for monitoring premature hearing loss in people on certain types of chemo. So if one would go to university hospitals you'd have a good chance of getting a UHF done, but you've found a way to do it anyways.
I'm just sort of baffled by the fact that they would have far more solid evidence of regeneration, and thus far more investor confidence and proof of effectiveness if they had only thought of UHF testing in their clinical trial. It was to be expected that the medicine would have to pass the apex of the cochlea first. Now they have a rather vague 10dB improvement at 8kHz which is fairly close to the margin of error of 5dB. Were they so bold to believe the results would be more favorable? Did they go "oops" after the trials were done?
Now we have to wait for phase 2 completion to get our answers.
You are correct. We await the completion of phase 2 as do the investors and Frequency's team to get clear answers to audiometric changes.
Fingers crossed.
They did not test UHF in phase 1. They are doing that in the current phase.So do we know if Frequency Therapeutics did hearing tests in the UHF region and whether they tested speech in noise?
The issue is in a phase one/phase 1/2 trial (what was done by Frequency Therapeutics) is that they are only allowed to propose putting a safe dose of a drug into a person to test that it is safe to consume. Hence no one should assume there would be high benefit demonstrated of a treatment in any of these phase one trials no matter the type of medication we are discussing.I wasn't aware it was hard to get UHF in the States.
UHF testing is routinely done for monitoring premature hearing loss in people on certain types of chemo. So if one would go to university hospitals you'd have a good chance of getting a UHF done, but you've found a way to do it anyways.
I'm just sort of baffled by the fact that they would have far more solid evidence of regeneration, and thus far more investor confidence and proof of effectiveness if they had only thought of UHF testing in their clinical trial. It was to be expected that the medicine would have to pass the apex of the cochlea first. Now they have a rather vague 10dB improvement at 8kHz which is fairly close to the margin of error of 5dB. Were they so bold to believe the results would be more favorable? Did they go "oops" after the trials were done?
Now we have to wait for phase 2 completion to get our answers.
The discussion around the success of the drug doesn't really even play into it at all. Frequency Therapeutics chose to see how it would work despite the drawbacks of the tests in a first trial by getting allowance to report the testing outcomes of the drug in the doses injected. Frequency Therapeutics proved two things from this trial. Firstly and most importantly that the drug was safe and secondly that the drug was doing what it was intended to do from both the fact that it would go into the ear and also that it assisted.
The testing that they used is the recognised testing in the USA which was where the trial took place. Thus the testing has to comply with these requirements and nothing else to be considered as being acceptable from the FDA. Frequency Therapeutics has no choice other than to comply if they want to meet FDA standards to get a medicine approved.
While skepticism is good as it imprints good discussion into topics, I think that you might be reading too much into the trial's results when stacked against its purposes. Thus now that Frequency Therapeutics has clearly proven that the drug is safe, they are allowed to try other methods such as staggered dosing and look at even higher dose sizes and alternative delivery methods in order to improve its outcomes. There is a high propensity to believe that there needs to be a bigger dosing delivered to give greater effect from the information in the initial trials. This is because it actually seemed that the higher dose people got greater benefit. Furthermore it also hints at why repeat dosing is being evaluated and why there is research into other dosing methods. All of these things though would not have been understood or recognised by Frequency Therapeutics had they not done this trial. Therefore this was quite beneficial.
All in all the trial evidenced that the drug does work and that the drug goes where it is wanted. We will need to wait until phase 2 is done or further research takes place probably to understand its wider benefit. What we can see to date is that the trial demonstrated that this drug works and what will need to be done further for Frequency Therapeutics to try and reform it to give better results.
Been posted here before but page 22-23 of this presentation has those results.What about speech in noise?
https://investors.frequencytx.com/static-files/6d161090-16f5-49f4-9606-8caceb5a88a1
They also are testing speech in noise in the current phase.
This and this there is no way that Frequency Therapeutics could have even easily determined the results of the trial testing. They proved pretty substantial things though which was the drug both entering the cochlear correctly and also being of a benefit to patients. Furthermore we saw the willingness and desire for Frequency Therapeutics to look at improving its efficacy. Expect evaluations to show more details in phase 2.The objective of the Phase 1/2 was to validate a safety assessment. The standard measures for hearing (words in noise, clarity, audiogram) were not even technically part of the official clinical trial. So, they probably had to work with what was available at the single testing site in San Antonio. They also encountered a ceiling effect having people with mild hearing loss.
Most Phase 1 or 1/2 don't provide anything close to the meaningful data gained from a handful of participants.
I don't see this as much of an "oops" as a refining of their strategy to validate the effectiveness of the drug within the scope of generally accepted clinical methods used today.
You always give very logical input. If I have high frequency hearing loss and high frequency tinnitus with hyperacusis, what might your opinion be on FX-322 helping with high frequency tinnitus? Looking for some hope.Been posted here before but page 22-23 of this presentation has those results.
https://investors.frequencytx.com/static-files/6d161090-16f5-49f4-9606-8caceb5a88a1
They also are testing speech in noise in the current phase.
A very good chance that FX-322 will help you. With the current formulation it is easier to repair high frequency hearing loss which should then get rid of your tinnitus and possibly hyperacusis.You always give very logical input. If I have high frequency hearing loss and high frequency tinnitus with hyperacusis, what might your opinion be on FX-322 helping with high frequency tinnitus? Looking for some hope.
You raise a good point about not expecting too much from a phase 1/2 trial as it's mainly for safety.While skepticism is good as it imprints good discussion into topics, I think that you might be reading too much into the trial's results when stacked against its purposes. Thus now that Frequency Therapeutics has clearly proven that the drug is safe, they are allowed to try other methods such as staggered dosing and look at even higher dose sizes and alternative delivery methods in order to improve its outcomes. There is a high propensity to believe that there needs to be a bigger dosing delivered to give greater effect from the information in the initial trials. This is because it actually seemed that the higher dose people got greater benefit. Furthermore it also hints at why repeat dosing is being evaluated and why there is research into other dosing methods. All of these things though would not have been understood or recognised by Frequency Therapeutics had they not done this trial. Therefore this was quite beneficial.
But, it's Frequency themselves that are using the positive results to attract investors. They knew they could only do one injection in one ear, so whatever gains they would see would likely be small. They could've guessed that and tried to document these gains as well as possible.
Now they have to go around with the data they did collect, and the results are ambiguous which is why we see some healthy criticism. After the recent failure of Audion it's only normal. Some good results don't always warrant a positive outcome.
I really believe the choice of not doing UHF testing was anything other than a deliberate one. Why? Was it that cost-prohibitive to get a UHF unit at the test site? Can't be...
Would they benefit from a somewhat vaguer and less documented result from an investor standpoint? Maybe.
Now we assume that the gains have been greater than 10dB at the higher frequencies because of how the drug is administrated. That's a logical assumption. But it can also be less. We have to keep that in mind.
If our tinnitus (or hearing loss) is in a region of great cochlear damage, there may be too little or no support cells to have any effect.
- Dec 18, 2015
- 619
- 46
- Tinnitus Since
- 03/2015
- Cause of Tinnitus
- Noise induced, loud rock concert
- Jan 5, 2020
- 90
- Tinnitus Since
- 2011, Spike 2019, Spike 2020
- Cause of Tinnitus
- 1e Loud Music, 2e earwax irrigation, 3e padel tennis
I have had a telephone call 2 months ago with the Medical Director of Astellas in The Netherlands. She has confirmed to me that there will be a clinical trial in Europe. Nothing more, nothing less. I have no clue when and where (probably not The Netherlands, because we have shit rules for testing medicines). But there will be one.I have seen posts about the possibility that Astellas might test FX-322 in Europe next year.
Does anyone know if there is any evidence for this, like a statement from Frequency or Astellas themselves or is it just speculation?
You would be a good candidate unless you have profound loss in those ranges (over 90 dB).You always give very logical input. If I have high frequency hearing loss and high frequency tinnitus with hyperacusis, what might your opinion be on FX-322 helping with high frequency tinnitus? Looking for some hope.
Do you have loudness hyperacusis or noxacusis?
Separate European trials under EMA supervision would make for a more compelling application for approval than only American-based, FDA supervised trials. That, and Frequency themselves stating there are payment milestones from Astellas to Frequency when the first patient is administered FX-322 in a European or Asian Phase 2b and Phase 3 trial. So I take it they've had discussions about European trials.I have seen posts about the possibility that Astellas might test FX-322 in Europe next year.
Does anyone know if there is any evidence for this, like a statement from Frequency or Astellas themselves or is it just speculation?
It is in agreement between Frequency Therapeutics and Astellas that the latter will conduct trials in other countries as well and in exchange Frequency Therapeutics gets cash capital.I have seen posts about the possibility that Astellas might test FX-322 in Europe next year.
Does anyone know if there is any evidence for this, like a statement from Frequency or Astellas themselves or is it just speculation?
I have noxacusis. Pain, burning, everything...You would be a good candidate unless you have profound loss in those ranges (over 90 dB).
Do you have loudness hyperacusis or noxacusis?
Hmmm profound. I don't know if it's profound. I just know it's in the high frequencies.
I had ganglion block the other day. No burning throat or ear on left, slight on right, increase in tinnitus. If I could get my tinnitus controlled, that is my main concern. It's still in my ears so I would like to get it treated before it takes over the whole head. If only I could get Frequency or Astellas to see that. I'd pay for the drug.
As far as next pain treatment I have is for occipital headaches and I have them injecting at the ear itself some blocks.
My biggest problem is tinnitus as it sets my whole body into fight or flight mode.
I do not have greater than 90 dB loss in standard audiogram but the decline is steep indicating movement toward that. For instance at 8 kHz I'm at 65 dB loss. When I tried doing the test for the higher frequencies at home, that's when I got hyperacusis.
I don't have a place I can live yet that is quiet enough. What did you do?
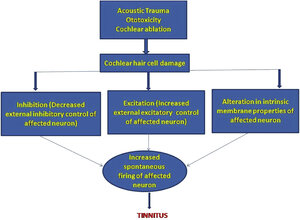
This makes perfect sense. Seems to be why many think that the treatment of tinnitus on its own isn't as good as hearing regeneration.Why regenerating functional hair cells looks so promising:
https://www.sciencedirect.com/science/article/pii/S0929664615003848
I'm not sure if drugs like FX-322 will help noxacusis, there isn't really data to show if the C fibers will get less sensitized after hair cells are repaired because previously that was not possible. These fibers elsewhere in the body can retain sensitization sometimes even when the original injury heals so that can be a concern for noxacusis. I think NAV receptor drugs offer possibly more promise there. But I'm really not sure. There is so little data on this, unfortunately.I have noxacusis. Pain, burning, everything...
Hmmm profound. I don't know if it's profound. I just know it's in the high frequencies.
I had ganglion block the other day. No burning throat or ear on left, slight on right, increase in tinnitus. If I could get my tinnitus controlled, that is my main concern. It's still in my ears so I would like to get it treated before it takes over the whole head. If only I could get Frequency or Astellas to see that. I'd pay for the drug.
As far as next pain treatment I have is for occipital headaches and I have them injecting at the ear itself some blocks.
My biggest problem is tinnitus as it sets my whole body into fight or flight mode.
I do not have greater than 90 dB loss in standard audiogram but the decline is steep indicating movement toward that. For instance at 8 kHz I'm at 65 dB loss. When I tried doing the test for the higher frequencies at home, that's when I got hyperacusis.
I don't have a place I can live yet that is quiet enough. What did you do?
Your tinnitus, especially being in a high frequency range stands a good chance of being helped. High frequency hearing loss that shows up on an audiogram, especially, points to hair cell loss usually (unless it's something like TMJ in more unusual presentations).
Not sure what you mean by "before it takes over the whole head." Do you mean yours is still very acute?
As far as "what did you do?". I don't have loudness hyperacusis any more and I just mask my tinnitus as much as I can. The sound distortions bother me more because loud masking doesn't make my tinnitus worse so I just have loud fans on all the time, etc. The worst thing for me is that my hearing loss/damage makes all music and media sound wildly distorted and horrifying.
- Feb 14, 2020
- 1,630
- Tinnitus Since
- 1-2019
- Cause of Tinnitus
- 20+ Years of Live Music, Motorcycles, and Power Tools
While people experience tinnitus in different ways. In the case of SNHL and NIHL, tinnitus is clearly a symptom of the underlying cause. So, there is no broad way to "cure" tinnitus since it is experienced from so many different things. This is what baffles me about the on-going tinnitus studies.This makes perfect sense. Seems to be why many think that the treatment of tinnitus on its own isn't as good as hearing regeneration.
In the case of what FX-322 is intended to repair (damaged cells from SNHL/NIHL), if the cells are replaced the tinnitus SYMPTOM that people experience may be reduced/eliminated.
Lets also keep in mind, the leadership at Frequency Therapeutics just did a podcast with Tinnitus Talk. I haven't heard it yet, but it seems promising if they're taking the time to talk to this specific community. Maybe it's a bold move; or maybe they have some qualitative insight?
- Jan 17, 2020
- 372
- Tinnitus Since
- 01/2020
- Cause of Tinnitus
- Probably noise, stress and a neck injury.
While I applaud any and all initiatives, this is what sometimes worries me. There's more causes of tinnitus but it seems like it's just not given any thought. I understand that they have to go with the most common, and probably most ''easy'' to fix though. Just hope that eventually everyone can have some relief. I guess it's good there's more research going on than ever, I just truly wish all of it would be focused on the mechanisms of tinnitus and how to treat/cure it and not whatever type of person can best deal with tinnitus. That doesn't help anyone.While people experience tinnitus in different ways. In the case of SNHL and NIHL, tinnitus is clearly a symptom of the underlying cause. So, there is no broad way to "cure" tinnitus since it is experienced from so many different things. This is what baffles me about the on-going tinnitus studies.
- Sep 21, 2016
- 1,051
- Tinnitus Since
- 2011 - T, 2016- H, relapsed 2019
- Cause of Tinnitus
- noise-induced
Agree with you - noxacusis seems to be a big unknown and it may well be better served by drugs that target the nerve fibers directly, like the new Retigabine or NaV 1.7 blockers. There's some interesting insights from the 2017 ARO event, at a conference hosted by Hyperacucis Research and the write-up from that included some discussion on the possible implications of regenerative approaches for hyperacusis (both loudness and pain) on pgs. 6-7: https://hyperacusisresearch.org/wp-content/uploads/2017/03/ARO-2017-Technical-Summary.pdfI'm not sure if drugs like FX-322 will help noxacusis, there isn't really data to show if the C fibers will get less sensitized after hair cells are repaired because previously that was not possible. These fibers elsewhere in the body can retain sensitization sometimes even when the original injury heals so that can be a concern for noxacusis. I think NAV receptor drugs offer possibly more promise there. But I'm really not sure. There is so little data on this, unfortunately.
Your tinnitus, especially being in a high frequency range stands a good chance of being helped. High frequency hearing loss that shows up on an audiogram, especially, points to hair cell loss usually (unless it's something like TMJ in more unusual presentations).
Not sure what you mean by "before it takes over the whole head." Do you mean yours is still very acute?
As far as "what did you do?". I don't have loudness hyperacusis any more and I just mask my tinnitus as much as I can. The sound distortions bother me more because loud masking doesn't make my tinnitus worse so I just have loud fans on all the time, etc. The worst thing for me is that my hearing loss/damage makes all music and media sound wildly distorted and horrifying.
I think one of the big unknowns is whether pain hyperacusis morphs into a self-perpetuating thing as you say so then hair cell regeneration may not be the way to go if the pain fibers retain sensitisation. But there's some interesting discussion there.

 Member
Member