Very informative podcast! My only concern was how far away OTO-6XX seems to be, but I've recently started wondering if hair cells have been over hyped.
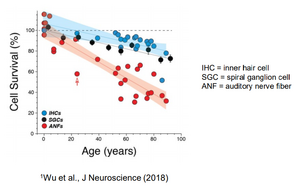
I wanted to share the below chart, which is found in Otonomy's latest slide deck:
View attachment 45322
I found this incredibly interesting. This is saying that most 80 year olds still have ~80% of their IHCs, but only around 40% of their synapses. Since FX-322 seems to target IHCs, this may be why that drug has been floundering in its latest studies. Additionally, this chart leaves me with the impression that hearing loss in old age is mostly due to synapse loss - however, this chart lacks OHCs, so maybe those play strong roll in hearing loss in old age?
I was curious if OTO-413 would regrow ANFs or just fix the synaptic connection, so I reached out to Otonomy for clarification and they responded:
In preclinical studies, BDNF has shown the ability to both regrow auditory nerve fibers and repair or re-establish their synapses with inner hair cells. As such, OTO-413 has the potential to restore auditory nerve fibers and their connections to inner hair cells in an 80 year old that has 40% remaining auditory nerve fibers to bring them back towards the normal level and improve hearing function.
That sounds really exciting to me. I also reached out to them about information regarding the audiograms of the patients in phase 1/2, since pig studies showed an 11dB improvement. They responded:
Thanks for the question. We are still doing standard audiograms as part of the safety assessment. For preclinical studies, we used auditory brainstem response (ABR's) because that's the only way to measure hearing function in animals. Since the primary mechanism of action of OTO-413 (BDNF) is repair of the neuronal connection, this is best assessed by evaluating speech-in-noise tests. That is why we focused on these tests in reporting a therapeutic effect in our proof-of-concept trial and why we are focusing on these tests going forward. We will report the audiogram results from a safety perspective.
I understand that audiograms are better for measuring OHCs and that they probably didn't see the needle move much here, but these systems all impact each other so part of me thinks we may see some improvement in this area. It'll be interesting to see what they report next summer.

 Member
Member Director
Director