I just think you seem overly obsessed with it when we still have no idea what it will do for tinnitus.
Must have something to do with every second of life being a living Hell and the fact that regeneration of hair cells is unprecedented through history. It doesn´t get much bigger than this.
Now, if it will alleviate or stop NIHL/SNHL induced tinnitus and hyperacusis we can still only make educated guesses.
In my opinion, it will. To me, it´s all about filling the "gaps".
We can talk about how complex this affliction is, and it is, because it involves our brain. And nothing is more complex than that, right?
Still the root cause, for most of us, is the ears' capability to transmit signals to the brain and the brain reacts to this in an effort to seek balance. In all neurological ways, the brain seeks balance.
So, why does someone develop tinnitus and some not?
Maybe I´m narrow-sighted and put too much of emphasis on my own case here, but I think it is a matter of "gaps" in our hearing. I am fortunate enough to show my upper end audiogram (8-16 kHz) and it really explains this "theory" in red and blue.
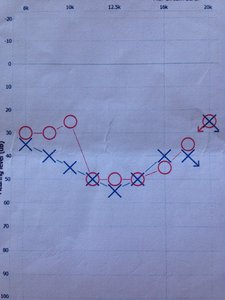
As you all probably know, red is right ear, blue is left.
As you can see, I have slightly more severe hearing loss in my right ear.
But do I have tinnitus in that ear? NO! Only my right ear, or on the right side of my brain, if you will.
The stern decline in my hearing "gap" (of 25 dB) must be the culprit then. It´s right there for all to see.
Also my tinnitus is matched to 12.5 kHz, right at the bottom of my decline in hearing of that ear. The loss of hearing on my left ear is more even, and the brain don´t feel the need to balance it out.
The reason for this btw is that I had a massive ball of wax in my left ear when I had my trauma, using headphones.

My hope, and belief is that Frequency Therapeutics drug can smooth this this out as FX-322 would know where it "hurts" and that my brain, probably as slow a process as it developed, will alleviate my tinnitus or even shut it the hell up... That's in line with what I know about brain plasticity.
Anybody find this interesting at all?
It certainly would be interesting to compare with others' "upper end" audiograms as I believe the evidence often lies there.
Sadly, very few has one.

 Member
Member


