What's the alternative? Is there even one on the horizon?For now... for now!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Frequency Therapeutics — Hearing Loss Regeneration
- Thread starter RB2014
- Start date
More options
Who Replied?Pero1234
Member
- Mar 15, 2018
- 287
- Tinnitus Since
- 02/2018
- Cause of Tinnitus
- home theatre system + high pressure washer
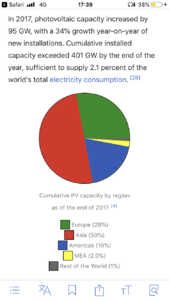
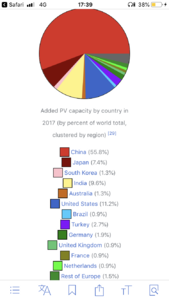
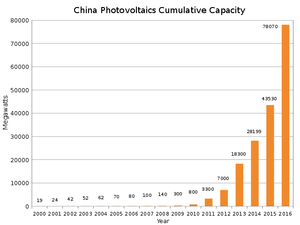
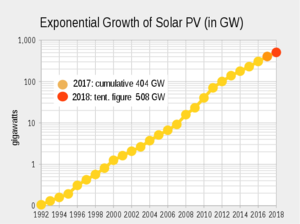
There are alternatives that are maturing... China is leading the pack. But still nowhere near enough to cover global energy needs.What's the alternative? Is there even one on the horizon?
But hey,
Rome was not built in one day...
Hearing cells were not regenerated in one day...
Energy transitions will take time... every step we take is one closer to the end goal.
If more people move to electric cars, the final switch will just become easier, right? Just connect another source to the grid and we're there!
A few graphs on solar in attachment.
Now... back to Frequency Therapeutics?
Attachments
Deathtotinni
Member
- Dec 21, 2017
- 157
- Tinnitus Since
- 9/10/17
- Cause of Tinnitus
- Qtip and maybe a sound..who knows
I've been inactive and trying to get used to my tinnitus. Stupidly went to fireworks... Did plug my ears but my tinnitus has since spiked. Though it could be due to a peptide I'm taking... Sooo I'm back.What's the alternative? Is there even one on the horizon?
What's the news with Frequency Therapeutics?
They said they saw good results from their last trial, no real details. They are going to run by a podium like Sonic the Hedgehog in September and say something about it into the microphone and then presumably start phase 2.I've been inactive and trying to get used to my tinnitus. Stupidly went to fireworks... Did plug my ears but my tinnitus has since spiked. Though it could be due to a peptide I'm taking... Sooo I'm back.
What's the news with Frequency Therapeutics?
Astellas Obtains the Exclusive Rights to Develop and Commercialize FX-322 in Ex-U.S. Markets; Frequency Retains U.S. Rights
Frequency will Receive $80 Million Upfront from Astellas with the Potential of up to $545 Million in Future Milestone Payments as well as Double-Digit Royalties
TOKYO and WOBURN, Mass., July 17, 2019 - Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., "Astellas" ) and Frequency Therapeutics, Inc. today announced that they have entered into an exclusive license agreement to develop and commercialize Frequency's regenerative therapeutic candidate, FX-322, for the treatment of sensorineural hearing loss, the most common type of hearing loss. At present, there are no approved therapeutic options for sensorineural hearing loss.
Under the terms of the agreement, Astellas will be responsible for the development and commercialization of FX-322 outside of the U.S. and Frequency will be responsible for U.S. development and commercialization. The companies will be jointly responsible for conducting global clinical studies and coordinating commercial launch activities. Frequency will receive an upfront payment of $80 million and may also receive up to an additional $545 million based on development and commercial milestones, as well as royalties on any future product sales in the licensed territory.
Frequency recently completed a Phase 1/2 clinical study in the U.S. in which FX-322 was observed to be well-tolerated following a single intratympanic injection, with no serious adverse events. Improvements in hearing function were observed in multiple FX-322 treated patients. Frequency plans to initiate a Phase 2a study in the fourth quarter of 2019.
Nice! Seems good news as an "efficacy indicator".View attachment 31010
Astellas Obtains the Exclusive Rights to Develop and Commercialize FX-322 in Ex-U.S. Markets; Frequency Retains U.S. Rights
Frequency will Receive $80 Million Upfront from Astellas with the Potential of up to $545 Million in Future Milestone Payments as well as Double-Digit Royalties
TOKYO and WOBURN, Mass., July 17, 2019 - Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., "Astellas" ) and Frequency Therapeutics, Inc. today announced that they have entered into an exclusive license agreement to develop and commercialize Frequency's regenerative therapeutic candidate, FX-322, for the treatment of sensorineural hearing loss, the most common type of hearing loss. At present, there are no approved therapeutic options for sensorineural hearing loss.
Under the terms of the agreement, Astellas will be responsible for the development and commercialization of FX-322 outside of the U.S. and Frequency will be responsible for U.S. development and commercialization. The companies will be jointly responsible for conducting global clinical studies and coordinating commercial launch activities. Frequency will receive an upfront payment of $80 million and may also receive up to an additional $545 million based on development and commercial milestones, as well as royalties on any future product sales in the licensed territory.
Frequency recently completed a Phase 1/2 clinical study in the U.S. in which FX-322 was observed to be well-tolerated following a single intratympanic injection, with no serious adverse events. Improvements in hearing function were observed in multiple FX-322 treated patients. Frequency plans to initiate a Phase 2a study in the fourth quarter of 2019.
So Astellas has global rights for development and commercialisation except for the US for which Frequency Therapeutics is responsible. I hope it will be truly global effort and not just Japan. But very good news and a strong validation for the technology by a large pharma that has seen all the data to date.View attachment 31010
Astellas Obtains the Exclusive Rights to Develop and Commercialize FX-322 in Ex-U.S. Markets; Frequency Retains U.S. Rights
Frequency will Receive $80 Million Upfront from Astellas with the Potential of up to $545 Million in Future Milestone Payments as well as Double-Digit Royalties
TOKYO and WOBURN, Mass., July 17, 2019 - Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., "Astellas" ) and Frequency Therapeutics, Inc. today announced that they have entered into an exclusive license agreement to develop and commercialize Frequency's regenerative therapeutic candidate, FX-322, for the treatment of sensorineural hearing loss, the most common type of hearing loss. At present, there are no approved therapeutic options for sensorineural hearing loss.
Under the terms of the agreement, Astellas will be responsible for the development and commercialization of FX-322 outside of the U.S. and Frequency will be responsible for U.S. development and commercialization. The companies will be jointly responsible for conducting global clinical studies and coordinating commercial launch activities. Frequency will receive an upfront payment of $80 million and may also receive up to an additional $545 million based on development and commercial milestones, as well as royalties on any future product sales in the licensed territory.
Frequency recently completed a Phase 1/2 clinical study in the U.S. in which FX-322 was observed to be well-tolerated following a single intratympanic injection, with no serious adverse events. Improvements in hearing function were observed in multiple FX-322 treated patients. Frequency plans to initiate a Phase 2a study in the fourth quarter of 2019.
They have facilities in the Americas, Asia/Oceania and Europe.So Astellas has global rights for development and commercialisation except for the US for which Frequency Therapeutics is responsible. I hope it will be truly global effort and not just Japan. But very good news and a strong validation for the technology by a large pharma that has seen all the data to date.
https://www.astellas.com/en/about/subsidiaries-and-locations
tarmaced
Member
- Mar 23, 2019
- 79
- Tinnitus Since
- 2004 (mild) 2018 (not so mild)
- Cause of Tinnitus
- Ear infections, compounded by noise exposure
View attachment 31010
Astellas Obtains the Exclusive Rights to Develop and Commercialize FX-322 in Ex-U.S. Markets; Frequency Retains U.S. Rights
Frequency will Receive $80 Million Upfront from Astellas with the Potential of up to $545 Million in Future Milestone Payments as well as Double-Digit Royalties
TOKYO and WOBURN, Mass., July 17, 2019 - Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., "Astellas" ) and Frequency Therapeutics, Inc. today announced that they have entered
I was wondering this as well? Let's say the benchmark is 100 million+ in funding injection and a partnership with a major pharma distributor on the basis of hair cell regeneration? Anyone know of this?Has any other candidate in the past arrived so far like this? Is this really a unique moment in the history of hearing? I hope so.
tarmaced
Member
- Mar 23, 2019
- 79
- Tinnitus Since
- 2004 (mild) 2018 (not so mild)
- Cause of Tinnitus
- Ear infections, compounded by noise exposure
My thought as well - I have 30 dB hearing loss so I should fall with a foot in the camp whichever way it wings for, high or low, rights XD.How much hearing loss does one need to have to be a candidate for the treatment though? I know I'm getting ahead of myself...
The pattern of cells remind me of honeycomb. I always imagine that, like bees, you may be better off starting with a pre-existing pattern. Fingers crossed it works for all though!
Chad Lawton
Member
- Mar 1, 2018
- 253
- Tinnitus Since
- 02/2018
- Cause of Tinnitus
- Possible Ototoxicity + Noise Exposure
I will fly to Japan if I have to!!!So Astellas has global rights for development and commercialisation except for the US for which Frequency Therapeutics is responsible. I hope it will be truly global effort and not just Japan.
Why go to Japan when you will be able to get it in the US?I will fly to Japan if I have to!!!
Staceyyy
Member
- May 25, 2018
- 48
- Tinnitus Since
- 3/102018
- Cause of Tinnitus
- SSHL from a kiss on the ear
This drug is my only hope, this is great news. I have 45 dB hearing loss in one ear with severe tinnitus and mild tinnitus in my other ear that does not have any sort of hearing loss. I hope this drug will help people without hearing loss as well. I still can't believe we haven't come across a single person who participated in the FX-322 clinical trials.
That's fantastic news.
My Dad was in pharmaceuticals for 40 years and now retired. He also has tinnitus. He doesn't expect any miracles, having helped bring many drugs to market, he knows the high rate of failure and length of time needed... but also told me he knows one of the investors in this drug and "he is no dummy" according to dad.
My Dad was in pharmaceuticals for 40 years and now retired. He also has tinnitus. He doesn't expect any miracles, having helped bring many drugs to market, he knows the high rate of failure and length of time needed... but also told me he knows one of the investors in this drug and "he is no dummy" according to dad.
US will probably take longer.Why go to Japan when you will be able to get it in the US?
I hope that this new licensing deal, $80M USD new capital and Astellas' contribution adds sufficient resources for the development of FX-322. Maybe they can speed up trials by adding Japanese and European facilities and patients. Somebody sent a link earlier stating Frequency Therapeutics has 14 employees. That has to be a major bottleneck in development. If you are convinced you have a billion dollar business you surely have more than 14 employees.
Statistically only about 30% of drugs that enter phase 2 make it to phase 3 so it's still better to be cautious. However, Astellas would not pay $80M USD for a drug candidate that is only safe. Instead, Astellas has been presented with strong data supporting efficacy of FX-322.
I am surprised that none of the tier 1 large pharmas like Pfizer, Novartis, Merck, Glaxo, etc made the deal. If it works, it is the biggest medical breakthrough in decades and someone will get a Nobel prize. And the market is so vast that it will be a blockbuster with sales in excess of 1 billion USD. Maybe the reason is that Astellas provided better conditions, valuation and royalty rates or that Frequency Therapeutics retaining US rights was not accepted by some.
Statistically only about 30% of drugs that enter phase 2 make it to phase 3 so it's still better to be cautious. However, Astellas would not pay $80M USD for a drug candidate that is only safe. Instead, Astellas has been presented with strong data supporting efficacy of FX-322.
I am surprised that none of the tier 1 large pharmas like Pfizer, Novartis, Merck, Glaxo, etc made the deal. If it works, it is the biggest medical breakthrough in decades and someone will get a Nobel prize. And the market is so vast that it will be a blockbuster with sales in excess of 1 billion USD. Maybe the reason is that Astellas provided better conditions, valuation and royalty rates or that Frequency Therapeutics retaining US rights was not accepted by some.
Understatement...Seems very promising if they got bought out by such a large pharma company.
It's awesome news friend... Yes.
Come on you nerdy capitalists... Make your millions... And help our ears.
The testing and research is outsourced and operates under their guidance. So there could be hundreds of scientists employed by them, working in third party labs.I hope that this new licensing deal, $80M USD new capital and Astellas' contribution adds sufficient resources for the development of FX-322. Maybe they can speed up trials by adding Japanese and European facilities and patients. Somebody sent a link earlier stating Frequency Therapeutics has 14 employees. That has to be a major bottleneck in development. If you are convinced you have a billion dollar business you surely have more than 14 employees.
Statistically only about 30% of drugs that enter phase 2 make it to phase 3 so it's still better to be cautious. However, Astellas would not pay $80M USD for a drug candidate that is only safe. Instead, Astellas has been presented with strong data supporting efficacy of FX-322.
I am surprised that none of the tier 1 large pharmas like Pfizer, Novartis, Merck, Glaxo, etc made the deal. If it works, it is the biggest medical breakthrough in decades and someone will get a Nobel prize. And the market is so vast that it will be a blockbuster with sales in excess of 1 billion USD. Maybe the reason is that Astellas provided better conditions, valuation and royalty rates or that Frequency Therapeutics retaining US rights was not accepted by some.
tarmaced
Member
- Mar 23, 2019
- 79
- Tinnitus Since
- 2004 (mild) 2018 (not so mild)
- Cause of Tinnitus
- Ear infections, compounded by noise exposure
This isn't an orphan drug though. The market is huge and to tap a real portion of that market they will need to compete with hearing aids...Since big pharma just got involved I can only assume that the treatment for this will be very expensive.
Have we got any evidence that restoring hearing will reduce tinnitus? Why then hearing aids don't help?
Hearing aids don't cover the highest frequencies (tinnitus is usually a very high frequency "beeeee"). So your brain keeps trying to compensate for the missing sounds.
- Apr 25, 2019
- 78
- Tinnitus Since
- 04/2019
- Cause of Tinnitus
- don't know, probably motorsport + headphones for last years
Could someone summarize the 139 pages of this thread to a few key points, what is FX-322 about?
What I understand and please, correct me If I'm wrong:
- FX-322 is a hearing restoration/regeneration drug.
- They just completed Phase I trial and this will be followed by a Phase II in the fourth quarter of 2019.
- They're about to announce "something" (results of Phase 1? anything else?) in the next weeks.
- They just got license agreement with Astella to both speed up the trials and possible commercialization.
I have mainly the following questions:
1. Is this just a single injection/shot directly into the inner ear?
2. Did they share any successes before? Any compliance/benefit numbers so far?
3. Some details, like how long does it take to see the results/improvements, is there any risk of worsening and other negative consequences, observations?
4. This might be suitable for people with measurable hearing loss, what about people with no hearing loss (clearly with hidden hearing loss?)
5. We don't know If hearing restoration/improvement helps to reduce/suppress tinnitus so far, right?
Is there a reason to be excited about this? Many previous experiments/attempts failed, right?
Noone from Trial here on the forum to share some experiences, right?
Many thanks for attempt to summarize this for me and potential new people into this!
What I understand and please, correct me If I'm wrong:
- FX-322 is a hearing restoration/regeneration drug.
- They just completed Phase I trial and this will be followed by a Phase II in the fourth quarter of 2019.
- They're about to announce "something" (results of Phase 1? anything else?) in the next weeks.
- They just got license agreement with Astella to both speed up the trials and possible commercialization.
I have mainly the following questions:
1. Is this just a single injection/shot directly into the inner ear?
2. Did they share any successes before? Any compliance/benefit numbers so far?
3. Some details, like how long does it take to see the results/improvements, is there any risk of worsening and other negative consequences, observations?
4. This might be suitable for people with measurable hearing loss, what about people with no hearing loss (clearly with hidden hearing loss?)
5. We don't know If hearing restoration/improvement helps to reduce/suppress tinnitus so far, right?
Is there a reason to be excited about this? Many previous experiments/attempts failed, right?
Noone from Trial here on the forum to share some experiences, right?
Many thanks for attempt to summarize this for me and potential new people into this!

 Member
Member