So what happens to the damaged hair cells? How do they get replaced?The new hair cells that are created using FX-322 do form synaptic connections. The original damaged hair cells that are still in place and have lost their synaptic connection before FX-322 administration will not form synaptic connections after FX-322 administration.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Frequency Therapeutics — Hearing Loss Regeneration
- Thread starter RB2014
- Start date
More options
Who Replied?Chad Lawton
Member
- Mar 1, 2018
- 253
- Tinnitus Since
- 02/2018
- Cause of Tinnitus
- Possible Ototoxicity + Noise Exposure
In their preclinical work, after dosing a mouse cochlea with FX-322, there were 6 rows of outer hair cells present rather than just 3 so this would suggest that dead/damaged hair cells will remain along side the new hair cells that FX-322 induces. It is not removing or repairing damaged hair cells, it is just generating new ones.And does that mean that, after therapy, the patient will end up with a bunch of damaged dead-weight hair cells grouped together with these brand new hair cells, competing for space?
- Sep 9, 2022
- 40
- Tinnitus Since
- 09/2022
- Cause of Tinnitus
- ultrasonic dental cleaning
That clears a lot of things up, thank you for the answer.In their preclinical work, after dosing a mouse cochlea with FX-322, there were 6 rows of outer hair cells present rather than just 3 so this would suggest that dead/damaged hair cells will remain along side the new hair cells that FX-322 induces. It is not removing or repairing damaged hair cells, it is just generating new ones.
If I may, one final question would be, then: why would it fail, then? If I recall correctly, the Phase 2 failed to achieve statistically significant results. Does that mean that, for some reason, the hair cells didn't grow in that trial, or that the new hair cells didn't help with hearing loss? Is there even a way to know which was the case?
Well, because a few things:If I may, one final question would be, then: why would it fail, then? If I recall correctly, the Phase 2 failed to achieve statistically significant results. Does that mean that, for some reason, the hair cells didn't grow in that trial, or that the new hair cells didn't help with hearing loss? Is there even a way to know which was the case?
1) Poorly designed original testing
2) If FX-322 is restoring any hearing, it is in 10 kHz+ range. It seems like clarity in hearing is in 8 kHz+ in addition to aid in helping hearing in noisy environments and this is what they were able to find out. This is why they check for speech perception.
Frequency Therapeutics are/were pioneers in the space and as such they are deemed to fail here and there but we clearly see now that they have learned from that experience and evolved.
FX-345 should have better results (as per Frequency Therapeutics) and reaching down to 4 kHz, so we will see.
I really hope if this fails they will let people try it for compassionate use. I think most of us would try it even if it has a 1% chance of helping. Intratympanic injections are safe too; nobody's had any adverse effects from any of the trials that I've heard of. I mean it's worth more of a try than spending $20,000 on stem cells.
I hope if the drug works even in the slightest bit, it gets approved. Because we all know audiology tests are an embarrassment, and many people have good audiograms and yet have bad tinnitus, hyperacusis, or distortions. So obviously we need better testing or a way to see inside that pesky cochlea.
I have been thinking more, is there any other organs you can't see inside at all from any imaging? Seems the cochlea is one of the only ones that are impossible to access and you can't see through the bone. Have to cut it out and dissect it.
I've read more interviews and we all saw how audiologists were saying a 5-10 dB improvement is negligible even if all patients see that improvement because audiograms vary between 5-20 dB from one test to another.
I'm glad Frequency Therapeutics understands 5-10 dB can be a big difference for some people, and can change the way one hears. I hope when they go for approval and that the garbage FDA doesn't give them any problems about that. I would lose my mind if it got denied because it didn't cure deafness lol. I hope the FDA understands that as well.
I hope if the drug works even in the slightest bit, it gets approved. Because we all know audiology tests are an embarrassment, and many people have good audiograms and yet have bad tinnitus, hyperacusis, or distortions. So obviously we need better testing or a way to see inside that pesky cochlea.
I have been thinking more, is there any other organs you can't see inside at all from any imaging? Seems the cochlea is one of the only ones that are impossible to access and you can't see through the bone. Have to cut it out and dissect it.
I've read more interviews and we all saw how audiologists were saying a 5-10 dB improvement is negligible even if all patients see that improvement because audiograms vary between 5-20 dB from one test to another.
I'm glad Frequency Therapeutics understands 5-10 dB can be a big difference for some people, and can change the way one hears. I hope when they go for approval and that the garbage FDA doesn't give them any problems about that. I would lose my mind if it got denied because it didn't cure deafness lol. I hope the FDA understands that as well.
@John Joseph, my understanding is that FX-345 has not even received industrial approval yet, let alone results from a clinical trial being available next year?with FX-345 results coming out next year.
Chad Lawton
Member
- Mar 1, 2018
- 253
- Tinnitus Since
- 02/2018
- Cause of Tinnitus
- Possible Ototoxicity + Noise Exposure
The largest reason it failed was due to an unprecedented placebo response. The issue isn't that no one responded, the issue is that too many responded in the placebo group. Both the treated group and the placebo group had statistically significant improvements but the company had to throw the baby out with the bathwater and say "it showed no benefit over placebo".That clears a lot of things up, thank you for the answer.
If I may, one final question would be, then: why would it fail, then? If I recall correctly, the Phase 2 failed to achieve statistically significant results. Does that mean that, for some reason, the hair cells didn't grow in that trial, or that the new hair cells didn't help with hearing loss? Is there even a way to know which was the case?
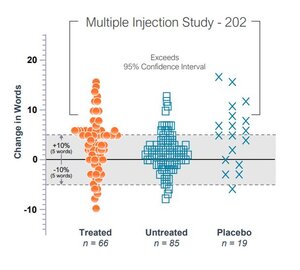
I've attached a screenshot showing the response rates for the FX-322-202 trial. The data points that fall within the 95% confidence interval bracket are the statistically significant responses. You can see that even some untreated ears had statistically significant responses which should not happen in a properly designed trial and validates the company's reasoning for why the trial failed.
The company attributes this to having only collected a single baseline measure at the start of the trial paired with some subjects lying to get into the trial by pretending they could hear less words than they actually could. One of the trial sites shared with subjects that they would need suppressed word recognition scores to get in and it was then shared on this thread here on Tinnitus Talk, thus opening the door for those desperate for tinnitus relief to try and get into the trial. In a post hoc analysis after the trial ended, the company got a hold of subjects historical word scores and found that many of the placebo subjects returned back to their historical average at day 90.
Another reason that may have contributed to the trial failing was them switching to multidosing. The company said they saw more of a muted response when multidosing vs single dose which is believable in my opinion (although it still produced statistically significant responses). They dosed 1x a week for up to 4 weeks in a row. Based on the mechanism of action of FX-322 and the fact it drives the progenitor cells to begin mitosis, it isn't surprising that telling brand new cells that are less than a week old to enter mitosis every week for 4 weeks in a row could potentially be detrimental rather than just letting the cells grow and do their thing with a single dose. The analogy I have used in the past is that its like baking a cake in the oven. How do you think your cake would turn out if you opened the over door every 10 minutes and added more flour and egg each time rather than leaving the door closed and letting the cake bake the way it's supposed to.
Attachments
@Chad Lawton, a well written piece. However, on the placebo response:
If people lied to get in the trials, then some would be in the intervention group and some in the placebo. So it would still even it out. Placebo responses can be 20-30% and a control group is essential for any trial. Look at the nerve block study and OTO-313. There may be 'something' - although a weak signal - in that graph you sourced. Thank you for posting that as it's the sort of thing I want to see.
I personally am not enthusiastic about this product, although looking at the 4000+ post thread, many are, but let's see.
If people lied to get in the trials, then some would be in the intervention group and some in the placebo. So it would still even it out. Placebo responses can be 20-30% and a control group is essential for any trial. Look at the nerve block study and OTO-313. There may be 'something' - although a weak signal - in that graph you sourced. Thank you for posting that as it's the sort of thing I want to see.
I personally am not enthusiastic about this product, although looking at the 4000+ post thread, many are, but let's see.
Chad Lawton
Member
- Mar 1, 2018
- 253
- Tinnitus Since
- 02/2018
- Cause of Tinnitus
- Possible Ototoxicity + Noise Exposure
To quote the company:@Chad Lawton, a well written piece. However, on the placebo response:
If people lied to get in the trials, then some would be in the intervention group and some in the placebo. So it would still even it out. Placebo responses can be 20-30% and a control group is essential for any trial. Look at the nerve block study and OTO-313. There may be 'something' - although a weak signal - in that graph you sourced. Thank you for posting that as it's the sort of thing I want to see.
"a review by the Company of available historical data from audiograms taken before the study, showed many study subjects with considerably lower baseline WR scores versus historical scores. This effect was observed among patients in all study groups. However, it was observed to be more frequent in the placebo group, and the level of WR scores at day-90 returned to values more consistent with historical data."
"Another observation of potential bias was also seen in inconsistent efforts by subjects in completing WR tests. Specifically, subjects could forego responding to test words in order to have a WR deficit at baseline. For example, one placebo subject, had 22 "no responses" on a 50 WR test given to the patient at baseline, while only 3 "no responses" were provided at the day-90 WR test."
"Another observation of potential bias was also seen in inconsistent efforts by subjects in completing WR tests. Specifically, subjects could forego responding to test words in order to have a WR deficit at baseline. For example, one placebo subject, had 22 "no responses" on a 50 WR test given to the patient at baseline, while only 3 "no responses" were provided at the day-90 WR test."
So the company says it occurred more in the placebo group than the treated group. Since there were over 20+ sites nationwide that participated, it's likely particular sites were worse offenders than others. To you it may be a weak signal, but for those in the studies who have managed to double their word scores; it is absolutely life changing.
You may not be excited for it but management still is. David Lucchino made these statements even AFTER the flopped Phase 2 during the 42nd Annual Goldman Sach's Healthcare Conference last year he said:
"Frequency Therapeutics has a clear signal. We have over 200 patients as a part of all of our clinical studies and numerous data points, and when we look at this data; even with the design issues that we had around our recent phase 2a, we continue to see a clear signal and continue to show confidence in this area."
"We are putting those learnings to work for FX-322, which we believe can be an approved product and beyond."
"A lot of ENTs & audiologists have been following our work closely. Both communities understand that with a therapy like this and we believe in when we commercialize it, its going to be a real shift."
"We are putting those learnings to work for FX-322, which we believe can be an approved product and beyond."
"A lot of ENTs & audiologists have been following our work closely. Both communities understand that with a therapy like this and we believe in when we commercialize it, its going to be a real shift."
And Chris Loose, their CSO said in April of this year during the B Riley Investor conference:
"We believe FX-322 can be the first regenerative therapy for hearing restoration"
Some may argue "well of course they are going to hype their own product, their jobs depend on it" but at this stage in the process they would have little reason to continue to waste time and resources on FX-322 if they didn't believe in it because they could easily just scrap it and put those resources to use on FX-345 instead. That is why I believe them when they say they believe FX-322 can be an approved product someday.
One other thing I forgot to mention that I think gets overlooked is that going into that Phase 2a that flopped, FREQ only had the small data set from their Phase 1/2 trial to try and pull data out of to pick their best potential patient populations to target in that Phase 2a. Now that they've had additional trials under their belt like the open label study, the age-related hearing loss study and the severe hearing loss study, they now have a much larger data pool to analyze to determine who exactly might respond and who might not and they are using all that data to load up the currently enrolling Phase 2b trial with patients they think will have the best chance of responding so that greatly helps their chances of success compared to the Phase 2a in my opinion.
@Chad Lawton, hmm yes, I can see where you are coming from a little bit more. Not enough to invest in it but fair points.
They will have to talk it up though at this point as I don't think they have anything else in human clinical trials. FX-345 is being filed for it now.
Fingers crossed.
They will have to talk it up though at this point as I don't think they have anything else in human clinical trials. FX-345 is being filed for it now.
Fingers crossed.
Not sure what you mean by industrial approval, but the first trial is supposed to begin in the fourth quarter of this year.@John Joseph, my understanding is that FX-345 has not even received industrial approval yet, let alone results from a clinical trial being available next year?
@John Joseph, my apologies if I'm wrong. Have they completed enrollment for Phase 1?the first trial is supposed to begin in the fourth quarter of this year.
roy1159
Member
- Feb 15, 2021
- 134
- Tinnitus Since
- 01/2020
- Cause of Tinnitus
- NIHL from working in a noisy cafe' for 2 years
Why the inclusion criteria doesn't take into account the 6 kHz threshold? It's as important as the frequencies up to 4 kHz in speech comprehension. Albeit I don't think it will affect the WR score after treatment because they demand a PTA of over 30 dB up to 4 kHz or somewhere in that range to enroll, which is quite a lot of damage and has significant effect on comprehension of vowels.
I always like reading your positivity backed by facts lol. Makes me feel a little better mentally that there's hope. I've always believed in this stuff and have kinda been losing hope lately. Let's hope this stuff becomes a reality.To quote the company:
"a review by the Company of available historical data from audiograms taken before the study, showed many study subjects with considerably lower baseline WR scores versus historical scores. This effect was observed among patients in all study groups. However, it was observed to be more frequent in the placebo group, and the level of WR scores at day-90 returned to values more consistent with historical data."
"Another observation of potential bias was also seen in inconsistent efforts by subjects in completing WR tests. Specifically, subjects could forego responding to test words in order to have a WR deficit at baseline. For example, one placebo subject, had 22 "no responses" on a 50 WR test given to the patient at baseline, while only 3 "no responses" were provided at the day-90 WR test."
So the company says it occurred more in the placebo group than the treated group. Since there were over 20+ sites nationwide that participated, it's likely particular sites were worse offenders than others. To you it may be a weak signal, but for those in the studies who have managed to double their word scores; it is absolutely life changing.
You may not be excited for it but management still is. David Lucchino made these statements even AFTER the flopped Phase 2 during the 42nd Annual Goldman Sach's Healthcare Conference last year he said:
"Frequency Therapeutics has a clear signal. We have over 200 patients as a part of all of our clinical studies and numerous data points, and when we look at this data; even with the design issues that we had around our recent phase 2a, we continue to see a clear signal and continue to show confidence in this area."
"We are putting those learnings to work for FX-322, which we believe can be an approved product and beyond."
"A lot of ENTs & audiologists have been following our work closely. Both communities understand that with a therapy like this and we believe in when we commercialize it, its going to be a real shift."
And Chris Loose, their CSO said in April of this year during the B Riley Investor conference:
"We believe FX-322 can be the first regenerative therapy for hearing restoration"
Some may argue "well of course they are going to hype their own product, their jobs depend on it" but at this stage in the process they would have little reason to continue to waste time and resources on FX-322 if they didn't believe in it because they could easily just scrap it and put those resources to use on FX-345 instead. That is why I believe them when they say they believe FX-322 can be an approved product someday.
One other thing I forgot to mention that I think gets overlooked is that going into that Phase 2a that flopped, FREQ only had the small data set from their Phase 1/2 trial to try and pull data out of to pick their best potential patient populations to target in that Phase 2a. Now that they've had additional trials under their belt like the open label study, the age-related hearing loss study and the severe hearing loss study, they now have a much larger data pool to analyze to determine who exactly might respond and who might not and they are using all that data to load up the currently enrolling Phase 2b trial with patients they think will have the best chance of responding so that greatly helps their chances of success compared to the Phase 2a in my opinion.
- Sep 9, 2022
- 40
- Tinnitus Since
- 09/2022
- Cause of Tinnitus
- ultrasonic dental cleaning
Thanks for the comprehensive writeup. Is there any way for people to have access to FX-322, under their own risk? If no, what is the earliest they could get access to it? Is the chemical structure of FX-322 known?To quote the company:
"a review by the Company of available historical data from audiograms taken before the study, showed many study subjects with considerably lower baseline WR scores versus historical scores. This effect was observed among patients in all study groups. However, it was observed to be more frequent in the placebo group, and the level of WR scores at day-90 returned to values more consistent with historical data."
"Another observation of potential bias was also seen in inconsistent efforts by subjects in completing WR tests. Specifically, subjects could forego responding to test words in order to have a WR deficit at baseline. For example, one placebo subject, had 22 "no responses" on a 50 WR test given to the patient at baseline, while only 3 "no responses" were provided at the day-90 WR test."
So the company says it occurred more in the placebo group than the treated group. Since there were over 20+ sites nationwide that participated, it's likely particular sites were worse offenders than others. To you it may be a weak signal, but for those in the studies who have managed to double their word scores; it is absolutely life changing.
You may not be excited for it but management still is. David Lucchino made these statements even AFTER the flopped Phase 2 during the 42nd Annual Goldman Sach's Healthcare Conference last year he said:
"Frequency Therapeutics has a clear signal. We have over 200 patients as a part of all of our clinical studies and numerous data points, and when we look at this data; even with the design issues that we had around our recent phase 2a, we continue to see a clear signal and continue to show confidence in this area."
"We are putting those learnings to work for FX-322, which we believe can be an approved product and beyond."
"A lot of ENTs & audiologists have been following our work closely. Both communities understand that with a therapy like this and we believe in when we commercialize it, its going to be a real shift."
And Chris Loose, their CSO said in April of this year during the B Riley Investor conference:
"We believe FX-322 can be the first regenerative therapy for hearing restoration"
Some may argue "well of course they are going to hype their own product, their jobs depend on it" but at this stage in the process they would have little reason to continue to waste time and resources on FX-322 if they didn't believe in it because they could easily just scrap it and put those resources to use on FX-345 instead. That is why I believe them when they say they believe FX-322 can be an approved product someday.
One other thing I forgot to mention that I think gets overlooked is that going into that Phase 2a that flopped, FREQ only had the small data set from their Phase 1/2 trial to try and pull data out of to pick their best potential patient populations to target in that Phase 2a. Now that they've had additional trials under their belt like the open label study, the age-related hearing loss study and the severe hearing loss study, they now have a much larger data pool to analyze to determine who exactly might respond and who might not and they are using all that data to load up the currently enrolling Phase 2b trial with patients they think will have the best chance of responding so that greatly helps their chances of success compared to the Phase 2a in my opinion.
I'm not sure if this has been posted before, but Frequency Therapeutics uploaded a new corporate presentation: https://investors.frequencytx.com/static-files/4e26405f-b117-4184-9740-5fdafc405bc6.
FX-345 Phase 1b results will be out in the first half of next year, other than that I don't think there's any new information for us.
For stock owners, they also talk quite a bit about their upcoming MS drug, for which they plan to start a Phase 1 trial next year.
FX-345 Phase 1b results will be out in the first half of next year, other than that I don't think there's any new information for us.
For stock owners, they also talk quite a bit about their upcoming MS drug, for which they plan to start a Phase 1 trial next year.
- Dec 17, 2014
- 164
- Tinnitus Since
- 11/2013
- Cause of Tinnitus
- Zithromycin acoustic trauma 2022
I'm not aware of the specifics on that. You can always try contacting them.@John Joseph, my apologies if I'm wrong. Have they completed enrollment for Phase 1?
This is a good question. I'm not well versed enough to know the answer (I'm not sure anyone really is when it comes to the mysteries surrounding this condition...), but it really boils down to whether the damaged cells themselves are the source of the tinnitus or the lack of auditory input is. If it's the latter, restoring hearing should at least help significantly.So this is great for people with hearing loss.
But if it grows new hair cells and doesn't connect the old damaged cells to the nerve, the one causing tinnitus, wouldn't the tinnitus remain?
Maybe better hearing will lower the tinnitus volume a bit...
Chad Lawton
Member
- Mar 1, 2018
- 253
- Tinnitus Since
- 02/2018
- Cause of Tinnitus
- Possible Ototoxicity + Noise Exposure
See their expanded access policy. FX-322 could be commercially available as early as the end of next year if the FDA accepts their current trial as pivotal. As for the chemical structure, you may be able to dig into their patents to find that out.Thanks for the comprehensive writeup. Is there any way for people to have access to FX-322, under their own risk? If no, what is the earliest they could get access to it? Is the chemical structure of FX-322 known?
Chad Lawton
Member
- Mar 1, 2018
- 253
- Tinnitus Since
- 02/2018
- Cause of Tinnitus
- Possible Ototoxicity + Noise Exposure
FX-345 data release has been pushed back to the 2nd half of next year per that slide deck. The most exciting tidbit in that updated slide deck is on slide 18 in my opinion. It states "Potential for FX-322-208 to be considered a pivotal study". This means they can potentially apply for FDA approval after this study and skip Phase 3. That would mean FX-322 could be commercially available by the end of next year if that were to happen.I'm not sure if this has been posted before, but Frequency Therapeutics uploaded a new corporate presentation: https://investors.frequencytx.com/static-files/4e26405f-b117-4184-9740-5fdafc405bc6.
FX-345 Phase 1b results will be out in the first half of next year, other than that I don't think there's any new information for us.
For stock owners, they also talk quite a bit about their upcoming MS drug, for which they plan to start a Phase 1 trial next year.
It's a common belief that the cause can be the lack of input. I wonder if it would work for noxacusis though since that's a bit different, being damage to an actual nerve probably. Only way to really know is to try it out.I'm not aware of the specifics on that. You can always try contacting them.
This is a good question. I'm not well versed enough to know the answer (I'm not sure anyone really is when it comes to the mysteries surrounding this condition...), but it really boils down to whether the damaged cells themselves are the source of the tinnitus or the lack of auditory input is. If it's the latter, restoring hearing should at least help significantly.
- Sep 9, 2022
- 40
- Tinnitus Since
- 09/2022
- Cause of Tinnitus
- ultrasonic dental cleaning
On that topic, is there any evidence the cause is the lack of input, rather than a continuous input?It's a common belief that the cause can be the lack of input. I wonder if it would work for noxacusis though since that's a bit different, being damage to an actual nerve probably. Only way to really know is to try it out.
That would be amazing. Probably need exceptional results for that. I'm happy enough if it just makes it to market, even if it takes another couple years.The most exciting tidbit in that updated slide deck is on slide 18 in my opinion. It states "Potential for FX-322-208 to be considered a pivotal study". This means they can potentially apply for FDA approval after this study and skip Phase 3. That would mean FX-322 could be commercially available by the end of next year if that were to happen.
Chad Lawton
Member
- Mar 1, 2018
- 253
- Tinnitus Since
- 02/2018
- Cause of Tinnitus
- Possible Ototoxicity + Noise Exposure
Seeing that there are no other treatments on the market, all they would need to do is show statistically significant improvements over placebo in my opinion.That would be amazing. Probably need exceptional results for that. I'm happy enough if it just makes it to market, even if it takes another couple years.
I'm pretty sure there isn't or else people would be sharing that information all the time and it would be used as a way of measuring effectiveness of drugs in clinical trials. Still lots of unknowns and there can be so many different causes of tinnitus too.On that topic, is there any evidence the cause is the lack of input, rather than a continuous input?
- Sep 9, 2022
- 40
- Tinnitus Since
- 09/2022
- Cause of Tinnitus
- ultrasonic dental cleaning
How is that still unknown, though? How hard should it be to just measure the output of the auditory nerve and check if it is continuously sending a signal or just turned off?I'm pretty sure there isn't or else people would be sharing that information all the time and it would be used as a way of measuring effectiveness of drugs in clinical trials. Still lots of unknowns and there can be so many different causes of tinnitus too.
Measure it how? What are you measuring? How do you differentiate signals and their meanings? I'm sure if it were easy then it would be known. There are a lot of medical issues that aren't fully comprehended by the medical world.How is that still unknown, though? How hard should it be to just measure the output of the auditory nerve and check if it is continuously sending a signal or just turned off?
- Sep 9, 2022
- 40
- Tinnitus Since
- 09/2022
- Cause of Tinnitus
- ultrasonic dental cleaning
Perhaps I'm being extremely dumb here, but aren't we debating whether damaged hair cells stop sending signals to the brain, or if they instead send a 24/7 signal? Couldn't that question be easily answered by simply measuring the nerve fibers that come out of the hair cells? I mean, specifically, here:Measure it how? What are you measuring? How do you differentiate signals and their meanings? I'm sure if it were easy then it would be known. There are a lot of medical issues that aren't fully comprehended by the medical world.
I don't see how such a thing could be unknown for so long. Either the nerve is firing, or it isn't, right? We know where the nerve is, and we have the tech to measure electric signals, so why can't we just measure it? Is it of hard access, or something like that? Am I completely off the track on my understanding?
Cynical viewpoint: If they abandoned FX-322, the share price would tank again and they're all heavily invested. Or maybe they really believe what they're saying? Hope so.they would have little reason to continue to waste time and resources on FX-322
Clinically significant I would have thought. No point to it if the effect is not worth the effort.all they would need to do is show statistically significant
It's all surrounded by bone. It is very small and delicate. No chance of being able to measure signals from individual hair cells. Some people got the auditory nerve severed and they still had tinnitus.Perhaps I'm being extremely dumb here, but aren't we debating whether damaged hair cells stop sending signals to the brain, or if they instead send a 24/7 signal? Couldn't that question be easily answered by simply measuring the nerve fibers that come out of the hair cells? I mean, specifically, here:
View attachment 51634
I don't see how such a thing could be unknown for so long. Either the nerve is firing, or it isn't, right? We know where the nerve is, and we have the tech to measure electric signals, so why can't we just measure it? Is it of hard access, or something like that? Am I completely off the track on my understanding?

 Member
Member