No. Read the paper.
Sorry. Wow awesome I am a lot more enthusiastic!!!
No. Read the paper.
Interesting...I didn't.
Consequently, it is necessary to inject the cells directly into the scala media, the fluid-filled lumen within the cochlear duct epithelium.
How does oval window delivery work? They remove the stapes bone?They are injected through the RWM or oval windows (or diffuse across the RWM) into the perilymph.
I think this is because the virus is released into the endolymph fluid, and it flows with the fluid, lands on the epithelium, sinks in and delivers the payload which starts to integrate with the supporting cells. The most successful of these so far, the ANC80L65 is specifically designed to withstand the potassium rich environment.the virus migrates throughout the inner ear
I believe so.Is it the basilar membrane that prevents them from migrating into the cochlear duct?
My understanding is that they drill a hole in the cochlea and inject directly into the scala tympani. This may vary by paper, but from http://www.nature.com/articles/srep46058When they say that they inject the cells into the scala media, do they enter via the scala tympani, through the basilar membrane?
Why would it be risky?I get that this is a amazing leap forward in terms of hearing restoration but using up your supporting cells in this procedure is risky especially you don't have a lot of hearing loss.
Why would it not get connected?My question is if a newly grown hair doesn't get connected up does it die.
How does oval window delivery work? They remove the stapes bone?
lands on the epithelium, sinks in and delivers the payload which starts to integrate with the supporting cells
The most successful of these so far, the ANC80L65 is specifically designed to withstand the potassium rich environment.
The virus would not be able to spread throughout the entire length of the epithelium.
My understanding is that they drill a hole in the cochlea and inject directly into the scala tympani.
I believe they drill a hole it it. This is the procedure for the Genvec/Novartis trial.
I am just speculating on potassium resistance. I don't have full access to that article.Again, this seems plausible though I don't think I have seem specific discussion of why it is that Anc80 is more effective across the full range of IHCs.
I can see why Auris, and many others are hoping that RWM diffusion will be good enough for therapy. All these other approaches are very risky.
What papers do you have in mind?I believe this is the approach professor Stefan Heller is working on.
No.Can we use viral vectors to deliver stem cells?
Doesn't matter. They diffuse through the RWM into the perilymph.Are they potassium compatible?
No.Can we use viral vectors for these molecules?
I assume this is the same type of procedure they use for stapedotomy when stapes footplate gets stuck. I read somewhere that they use 0.7 mm microdrill or laser to make the hole. But in stapedotomy and stapedectomy, they don't drill the hole all the way through.
What kind of procedure did you have? Did they remove your stapes arch and footplate?They do. How else is the prosthesis going to act as a piston and move freely in the hole?
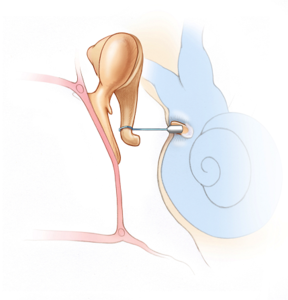
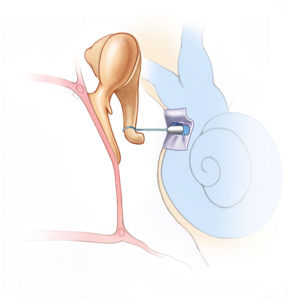
That sounds like you had stapedectomy?There's only a sleeve that is inserted between the piston and the hole, which is made from a graft.
Vein from your hand? I'm not sure what that means. Could you explain?For me they took a vein from my hand.
It will do that, the piston becomes part of that chain. But I don't think need an open hole in the bony labyrinth. You just need to shake the footplate at the oval window. This may be done a little differently, but I am pretty sure they don't drill a hole all the way through. What good would that be? The piston would just move in and out. It needs to grab hold of the footplate, if it's still present, or the surgical graft and shake it back and forth.The piston has to be able to transmit the mechanical vibrations from the ossicular chain into fluid waves in the cochlea.
I still don't think they intentionally want to or need to poke a hole all the way through. What you describe may be the result of a partially failed procedure, where they just went in too much. They need to stop drilling 0.5 mm before they poke through. That's not so easy to achieve, it's a microsurgery. So I am thinking that they might have gone a little bit too deep.That's actually one of the risks with the procedure. For people who have endolymphatic hydrops, there can be extra pressure from the fluid in the cochlea which results in a "gusher" when they finally poke through. It's not good when that happens.
 It's just something I have read about briefly, these different surgeries of the middle ear.
It's just something I have read about briefly, these different surgeries of the middle ear.What kind of procedure did you have? Did they remove your stapes arch and footplate?
That sounds like you had stapedectomy?
Vein from your hand? I'm not sure what that means. Could you explain?
It will do that, the piston becomes part of that chain. But I don't think need an open hole in the bony labyrinth. You just need to shake the footplate at the oval window. This may be done a little differently, but I am pretty sure they don't drill a hole all the way through. What good would that be? The piston would just move in and out. It needs to grab hold of the footplate, if it's still present, or the surgical graft and shake it back and forth.

The conductive type of otosclerosis usually progresses up to a maximum when patients are in their 30s. After this, it rarely progresses. Dizziness or imbalance is a feature of otosclerosis in roughly 25% of cases. Between 40% and 65% of patients have tinnitus (ringing in the ears) (Gristwood & Venables 2003, Sobrinho et al 2004). In about half of all patients, there is a family history of similar problems. Tinnitus appears to improve following surgical intervention in a number of cases, since "the conductive hearing deprivation produced by otosclerosis is associated with limited and reversible modifications in the central auditory pathway that are linked to tinnitus"(Deggouj et al 2009).
I am starting to think Gen-therapy will be suited better for inner ear regeneration than stem cell therapies. At this moment in time that is.
@GregCA Thanks for sharing! I know this thread is about hair cells and nerves and such, but this is very interesting too!
How was your hand after this? Did it heal up nicely?
How was your hearing after the surgery? How much did it improve?
What is it about otosclerosis that causes tinnitus? It can't possibly damage the hair cells?
If you guys want to have an in-depth discussion about stapedectomy/stapedotomy, it would make sense to start a new thread (unless there is an existing one).

This outer hair cell gets thinner when it gets longer and fatter when it gets shorter. Measuring up these changes indicates that the cell volume stays constant. This supports the idea that the 'motor' is a molecule whose job it is to change membrane area. The molecule, discovered in 2000 by Peter Dallos' lab in the US, is called 'prestin'.
Since the target was spiral ganglion neurons, the cells were not injected into the inner ear so potassium was not an issue.Can you discuss how the paper describing injected stem cell death within the ear matches some of the prior success Dr. Rivolta has had with neuron stem cells? Would neuronal stem cell for nerve repair and synaptic regrowth have to travel through the high K environment? Did he just lose a lot of stem cells and make up for that in terms of quantity?
We could all speculate, but it would not, as far as I know, be based on any published research and so there would be little or no point.Can anyone speculate on the effects of multiple viral injections of the AAV or ANC-80? Would the immune system react to a second injection? Has this been tried in animals?
Nonetheless, I am far more skeptical about this paper than many people here.
What exactly are those questions?I dont think your wrong because stem tech has been around long enough that more of these questions should have been answered.
Don't worry, the US is not the only one researching this. Besides, didn't Trump appoint that pro-biotech guy as the head of FDA? He is for progressive approval of new drugs. That's a good thing, I think.I know the US has stifled research.
Type 1 or type 2? If you can generate new ones, then there should not be a problem. If you can't, then it's probably more advantageous to damage Type 1 neurons if your main problem is bad hearing, or Type 2 neurons if your main problem is tinnitus. Type 1 is likely more responsible for tinnitus than Type 2.Still if you have lost or damaged beyond repair some SGNs would there be another to replace them?
Don't worry, the US is not the only one researching this. Besides, didn't Trump appoint that pro-biotech guy as the head of FDA? He is for progressive approval of new drugs. That's a good thing, I think.

Apparently it is difficult if not impossible at this moment in time to get stem cells to survive in the inner ear.What made you come to this decision?
Do they have to get to the inner ear to be effect for neuron regrowth? Could/do the majority of stem cells injected into the middle ear make it to the SGN? If they do how much type 1 and type 2 neuronal regrow results? If at this time stem cell injected into the middle ear are not useful for hair cell regrowth but do create type 1/2 nerve fibers then for tinnitus that is the whole ball game and we should be happy that there is a different treatment option. If not than I agree stem cell are a long way off from being useful for hearing restoration.Apparently it is difficult if not impossible at this moment in time to get stem cells to survive in the inner ear.