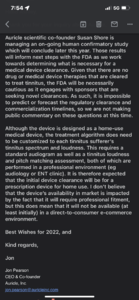
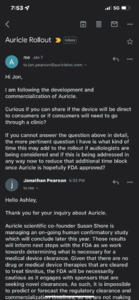
Hi all,
Just got confirmation from Jon Pearson that the device will most likely be prescriptive due to the pitch matching assessment so we will see this distributed in a clinical setting if it makes it to market.
I respect everyone's opinions and thoughts whether you are for or against contacting business and research members about the project. I'm in a highly distressed state with the recent onset of chronic reactive HF tinnitus so I am beyond being concerned about the opinions of others at the moment. Hope this provides some answers and comfort to anyone who is seeking them.
Much respect to
@Survivor234 for the insight you have been able to gain and share while remaining respectful and humble in response to all the criticism that has been sent your way. The lack of compassion from some of the responders has been downright disheartening.
Minor tidbit - I like the branding and marketing as far as the logo goes.

 Member
Member