- Feb 14, 2020
- 1,630
- Tinnitus Since
- 1-2019
- Cause of Tinnitus
- 20+ Years of Live Music, Motorcycles, and Power Tools
Your concerns are unwarranted. The trial ends in June, and June hasn't even ended. These Phase 1 trials almost always go past the expected completion date, sometimes by many months. Even though its an effective treatment/assessment window that lasts 3 months, it's really more like 4-5 which includes a lead-in for scheduling the first dosage and variances in the follow-up appointments. Recruiting also takes more time than you think, because there are specific guidelines the FDA has the testing practices follow. For example, they cannot market to patients. The patient has to be in the office for a regular visit when asked to participate in a study. I would probably be a candidate given the requirements of this study... but I haven't been to my ENT in almost 1.5 years; so the stars would have to align.I've been digging around trying to find clues about how the results of this study may turn out. I found an article from last year that had an interesting quote from their CEO: [1]
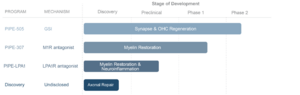
The dual mechanism of action for PIPE-505, involving repair of the cochlear synapse and the regeneration of outer hair cells critical for hearing quality and sensitivity, uniquely positions this small molecule to address two of the main cochlear elements commonly lost in SNHL
The chart I showed in the OTO-413 thread indicated that the loss of synapses and OHCs seem to be the main reasons for age related hearing loss (and SNHL too). Since this drug addresses both of those, I wonder if we could see blow out results from this first study?
However, I have some concern that it's taking them so long to release the top-line results. In that article they were expecting to release the top-line results "early 2021". Their study is small, involving only 24 patients and 90 days. I can't imagine recruitment was that hard. Additionally, they raised money in February, which I find concerning. If they were confident in the results, why not wait until afterwards to raise money? Surely they'd get a better deal if they had good results.
Also, I saw that they were delivering the drug via an intratympanic injection. Anyone know if they have a special time-release gel like Otonomy?
[1] Pipeline Therapeutics Initiates Phase 1/2a Clinical Trial of PIPE-505 in Sensorineural Hearing Loss
Also, there's consent of the patient, "Hey we're going to inject this experimental drug into your already damaged ears, if it fucks your hearing up, sorry..." How would you approach this? Would it be beneficial if you knew there's a risk this stuff could make you worse off in the name of science?
There's a lot of work that has to go into the analytics after the readout. They need to be exact and clear, so they'll want to take an appropriate amount of time with the readout. Since they're not publicly traded, they're not obligated by the SEC / FDA to release readouts within a reasonable timeframe.
My own expectations are at the very least, they'll take until late July, early August go provide anything tangible from the PIPE-505 trial.
Link to trial page:
Phase I/IIa Study Evaluating Safety and Efficacy of an Intratympanic Dose of PIPE-505 in Subjects With Hearing Loss

 Member
Member