A Personal Appeal From Myself Regarding Misinformation...
This week, and also the last one, has seen some sad news: The Autifony Therapeutics QUIET-1 study was terminated prematurely due to lack of efficacy. Less noticed to many - probably - was the news regarding Otonomy and their upcoming OTO-311 phase-I trial - which - has now been confirmed, but only for healthy volunteers (not patients). In addition, a media attack was launched on SciFluor Life Sciences by the hedge fund Kerrisdale Capital - which may have implications down the road. All-in-all, not a good past week for the worldwide tinnitus community.
With the above in mind, it saddens me to read misinformation from various random posters regarding the AM-101 phase-III trial. Right now, and for the foreseeable future, there is really only one pharma company left to help the tinnitus community: Auris Medical AG. It does the entire tinnitus community a disservice when posters feel the need to post seemingly randomly picked information to the effect of "tinnitus is a 'brain thing', therefore AM-101 will not work" and "there is a risk of permanent hearing loss if you enroll in the trial". Speculation has never helped anyone. Leave out the fiction, and stick with the facts.
In terms of facts, Auris Medical and physicians involved in the trial have already willingly spent their time addressing questions from members of TinnitusTalk. These Q&A-sessions can be found here:
In addition, there is an information page related to the AM-101 study which can be found here:
www.tinnitus-study.info
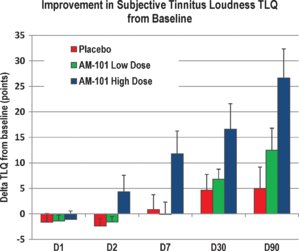
Lastly, I will attach various peer reviewed papers on the AM-101 study. If you want facts, then read those - not the all the hearsay and background chatter.
Misinformation can sometimes influence the decision a person makes as to whether to enroll in a trial. It would be sad that a person decided against enrollment based on the wrong information only to endure life-long tinnitus. So again, and in the interest of everyone, stick with the facts.
I hope that there will be ambassadors of this forum willing to ensure that facts, not fiction, dominate the decision making in this thread. Thank you.
attheedgeofscience
14/OCT/2015.
Tags:
@SoulStation @billie48 @OddV

 Member
Member