You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
AM-101 TACTT1 Results Released
- Thread starter Hudson
- Start date
More options
Who Replied?4 months is alot of time for the body to recover or get into another stage of a proces... I mean that if something like chronic and accute tnnitus can be seperated it has to be because the body moved into a diffrent level and 4 months is more than enough for the body to do so ... let`s keep the fingers crossed and wait for the next person saying he had it 12 months and is fully cured!! exiting ...
btw Erlend, like your devotion to finding information about this and sharing it!
btw Erlend, like your devotion to finding information about this and sharing it!
I am just curious. Does anyone know how the more "chronic" people handled the drug. Did it help out? I see A.M. did reduce the time threshold for the p1 study for the p2 study. Was it because they were having no results with the "chronic people". Or is it that they were having better results with people who have just contracted tinnitus and want to keep up good results for the FDA.
I dont think they tested this on chronic people yet...? Thats what the next phase is too see how it affects the 3 to 12 month stage.I am just curious. Does anyone know how the more "chronic" people handled the drug. Did it help out? I see A.M. did reduce the time threshold for the p1 study for the p2 study. Was it because they were having no results with the "chronic people". Or is it that they were having better results with people who have just contracted tinnitus and want to keep up good results for the FDA.
I am just curious. Does anyone know how the more "chronic" people handled the drug. Did it help out? I see A.M. did reduce the time threshold for the p1 study for the p2 study. Was it because they were having no results with the "chronic people". Or is it that they were having better results with people who have just contracted tinnitus and want to keep up good results for the FDA.
Where does this persistent myth that they reduced the time frame keep coming up from? Do you have a link to this? Hell, the founder of Auris Medical sent some emails responding to one of our threads once because another guy was claiming the timeline had been reduced. As far as I can tell, for all of their clinical trials, AM-101 has had an onset window of 3 months. Actually, for a couple clinical trials in the phase 3 realm, they have expanded the treatment window to 12 months.
Because they are starting with what they view to be the easiest and most likely candidates to respond to treatment, and after they get approval for the drug and start making boat loads of money, they'll probably expand their treatment audience.I do not see what the value is to test on non-chronic patient (under a year of T).. The ear can take 5-6 months to recover naturally so how can a company tell what is natural and what is from the drug.
I have already posted this somewhere else but you may find it interesting. It is in French but google translate works well: http://www.france-acouphenes.org/forum1/viewtopic.php?t=10658&start=0
It is from someone who has tested the AM-101 in 2009 and got a partial relief. He said his tinnitus was "more central, softer and much more bearable"
From memory the reason for the short time window was that AM-101 targeted "otic" tinnitus, they thought it was at first an abnormal activity of the auditory nerve and after several months it became more of a brain thing where the AM-101 cannot act. That is also why they think you must have some sort of auditory damage for the drug to work. Tinnitus not coming from the ear would not be a target for this drug.
It is from someone who has tested the AM-101 in 2009 and got a partial relief. He said his tinnitus was "more central, softer and much more bearable"
From memory the reason for the short time window was that AM-101 targeted "otic" tinnitus, they thought it was at first an abnormal activity of the auditory nerve and after several months it became more of a brain thing where the AM-101 cannot act. That is also why they think you must have some sort of auditory damage for the drug to work. Tinnitus not coming from the ear would not be a target for this drug.
I do not see what the value is to test on non-chronic patient (under a year of T).. The ear can take 5-6 months to recover naturally so how can a company tell what is natural and what is from the drug.
This is the power of the double blind placebo study. They give the drug to one group of people and a placebo drug to another group. Neither of the groups know what they got (double blind). Then they have to find the difference in cure rate (here probably som kind of reduction parameter) between the two groups to be statistically significant in order to claim the study to be succesful.
Do you know already when you will get your first injection?I have just returned from my AM-101 trial screening. The trial will consist of 3 injections. Being double blind, there is a 30% chance that I will receive a placebo. The doctor that I talked to was extremely knowledgable about the procedure and has very high hopes for the future of this drug. After I officially enroll on Monday, I will be scheduled an appointment for my first injection. As I said before, I will keep you updated through the entire process and feel free to ask me any questions.
I read the French Article posted a few posts above me and both people stated in the end that Am 101 did not help them out or eluded that it did not help them out to much. Orginally the male who got the drug (I do not think he knew if he got the drug or the placebo) said it helped in the beginning. A month later he said, his T kinda came back.
Also it said that the people that partcipated in P1 and P2 were subject to a quasi gag order, which makes sense given that there is not to much talk from participants found on the web.
Then the moderator shut the forum down, which is terrible because no one got to know their specific ending of their story, which would not be significant of anything, however it would be interesting to know.
Also it said that the people that partcipated in P1 and P2 were subject to a quasi gag order, which makes sense given that there is not to much talk from participants found on the web.
Then the moderator shut the forum down, which is terrible because no one got to know their specific ending of their story, which would not be significant of anything, however it would be interesting to know.
I emailed autifony and asked a few questions regarding chronic tinnitus and
How the time this drug hits the market ( if it does) that all of us will have chronic t by then and if he still thinks am 101 will be effective... I know this has already been posted a hundred times and i know that no ones gonna have a direct answer.. And i also asked about trials near me and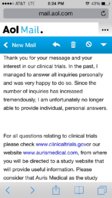
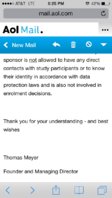 they sent me this.. Sorry if it looks weird not good with uploadin.
they sent me this.. Sorry if it looks weird not good with uploadin.
How the time this drug hits the market ( if it does) that all of us will have chronic t by then and if he still thinks am 101 will be effective... I know this has already been posted a hundred times and i know that no ones gonna have a direct answer.. And i also asked about trials near me and

 they sent me this.. Sorry if it looks weird not good with uploadin.
they sent me this.. Sorry if it looks weird not good with uploadin.I emailed autifony and asked a few questions regarding chronic tinnitus and
How the time this drug hits the market ( if it does) that all of us will have chronic t by then and if he still thinks am 101 will be effective... I know this has already been posted a hundred times and i know that no ones gonna have a direct answer.. And i also asked about trials near me and View attachment 832 View attachment 833 they sent me this.. Sorry if it looks weird not good with uploadin.
Thanks for the effort. Do you know if you have to be a resident of Germany, Austria or Uk in order to participate in the study?
Thanks for the effort. Do you know if you have to be a resident of Germany, Austria or Uk in order to participate in the study?
I think they only take citizens / natives of the countries were trials are held. Also maybe residents, but I am not sure about it. If you live in another country, they won't allow you to participate for safety reasons.
no not yet
Good luck, Jake! Keep us posted.
We also have a trial site here in Miami, and I was on the list of potential subjects. But I didn't make the cut because I am nine months from onset, and they are sticking to the three-month window here in the US. For now. Have heard they may expand the onset criteria in the future here, as they did in the EU (as @Hudson said, they will want the largest potential market possible).
I also am enthusiastic about AM-101's potential... but hope we all aren't pinning too many hopes on it. Maybe I am being overly cautious. But I remember all the hoopla when Aricept came on the market for Alzheimer's patients. However over time and extensive use, we have seen that while Aricept can initially stall Alzheimer's symptoms, it ultimately does not work long-term... and does not work on many patients at all.
Having said that, I am up for any (safe) help the pharmaceutical industry might throw me, and would have gladly participated in the trial. But I also am continuing to work on my T management skills, given I don't know what AM-101 or any other experimental treatment will ultimately lead to.
Thanks for the effort. Do you know if you have to be a resident of Germany, Austria or Uk in order to participate in the study?
You have to be a resident and a native speaker of the language where they have it
I emailed autifony and asked a few questions regarding chronic tinnitus and
How the time this drug hits the market ( if it does) that all of us will have chronic t by then and if he still thinks am 101 will be effective... I know this has already been posted a hundred times and i know that no ones gonna have a direct answer.. And i also asked about trials near me and View attachment 832 View attachment 833 they sent me this.. Sorry if it looks weird not good with uploadin.
You emailed Autifony about Am101....I assume you mean Auris Medical??..confused
Yeah lol sorry totally typed that wrong!You emailed Autifony about Am101....I assume you mean Auris Medical??..confused
Ha now that you mentioned that I confused myself when writing that email.. Oh well was pointless cause they still cant write back personal answers...Yeah lol sorry totally typed that wrong!
Lady Di, I think the US sites are getting people who have had tinnitus from 3.1 months to 12 months. There also taking people under 3 months. I am pretty certain. Maybe Florida's sites are only for people under 3 months. I do not know.
- Jul 21, 2013
- 842
- Tinnitus Since
- 01/2013
- Cause of Tinnitus
- Acoustic trauma from headphones
Good luck, Jake! Keep us posted.
We also have a trial site here in Miami, and I was on the list of potential subjects. But I didn't make the cut because I am nine months from onset, and they are sticking to the three-month window here in the US. For now. Have heard they may expand the onset criteria in the future here, as they did in the EU (as @Hudson said, they will want the largest potential market possible).
I also am enthusiastic about AM-101's potential... but hope we all aren't pinning too many hopes on it. Maybe I am being overly cautious. But I remember all the hoopla when Aricept came on the market for Alzheimer's patients. However over time and extensive use, we have seen that while Aricept can initially stall Alzheimer's symptoms, it ultimately does not work long-term... and does not work on many patients at all.
Having said that, I am up for any (safe) help the pharmaceutical industry might throw me, and would have gladly participated in the trial. But I also am continuing to work on my T management skills, given I don't know what AM-101 or any other experimental treatment will ultimately lead to.
Well said. AM-101 is absolutely a step in a the right direction. At this point, ANYTHING is better than what we have. It may or may not work on long-term sufferers. We just don't know. Time will tell. In either scenario, we will have even more treatments being developed, until we have full inner ear restoration & regeneration.
Hey Mark: I can tell you for certain that Miami only is taking people at three months or under, at least at this point. I thought the rest of the US sites were the same, but could not tell you for sure. And I have not reached out to Auris myself. I already had decided I was not willing to travel far to be part of this study, so I looked no further.
But I would be interested in what others have to say.
And @Champ, I agree: Anything is better than what we have now. Plus an AM-101 success encourages other med tech and drug companies to look for their own tinnitus treatments. I just worry that some people think AM-101 will be "curing" us all within the next few years -- and I don't know (but of course, have no way of knowing for certain, I could be wrong) if that will be the case. As I have said before, I think tinnitus will be a very tough treatment nut to crack. Just guessing, but my guess is even if its successful, AM-101 will work on a select group of patients, the variables being time from onset, how the patients got their T, and other things.
But I would be interested in what others have to say.
And @Champ, I agree: Anything is better than what we have now. Plus an AM-101 success encourages other med tech and drug companies to look for their own tinnitus treatments. I just worry that some people think AM-101 will be "curing" us all within the next few years -- and I don't know (but of course, have no way of knowing for certain, I could be wrong) if that will be the case. As I have said before, I think tinnitus will be a very tough treatment nut to crack. Just guessing, but my guess is even if its successful, AM-101 will work on a select group of patients, the variables being time from onset, how the patients got their T, and other things.
Hi!
I just registered because I was reading this post about the TACTIII study here. You guys seem pretty curious and positive about it. I wasn't sure wheter to participate or not. Anyway I did it...
I'm from Germany and I have tinnitus since october 2013. It was going better until I reached
a level that I could live with. Then one weekend in January I went out to party (with propper earplugs of course) and ended up with a really horrible worsening. I decided to participate in the Am101 study. The worsening went away and I wasn't sure wheter I
should participate or not. I did because it semms pretty safe to do so. The doctor had done the AM101 studies before and she is also pretty porisitve about it. The drug Am101 is Ketamine, which is used and pretty safe. The method of application is: horrible but said to be pretty safe. First they numb your eardrum and then suck the numbing-liquid back out with a aspirator, that makes a horrible loud noise. Then you got a hole poked in it, which makes a horrible sound and is quite painfull. Then you get your inner ear filled with a liquid, that stays there for a coulple of days. On the second shot my eardrum was pretty sensitive and the injection was really painfull altough it got propper numbed. The doctor said, she thinks I got the real drug, because she thinks the drug made my eardrum more sensitive to pain and a placebo would not have done that. Besides she got a proband with the same issues before and he got the real drug. She needed to poke a new hole in my eardrum everyday, because it closed everytime. .. Ahmm.. decided to get it done just on one ear, although I got tinnitus on both ears. For people like us, with pretty 'sensitive' ears, that try to take care of them as much as possible, this procedure a real horror and pretty scarring. absolutly nothing for people with hyperacusis!
I got the three shots on monday, tuesday and wednesday. My right ear is still filled with that stuff, which I don't know if it's placebo or not. My tinnitus is a lot worse and my hearing changed because of the liquid that is still in my ear. I hope the liquid will run through the eustachian tube soon and leave me tinnitus free!!
This is my story with TACTIII so far, if you got any questions, please ask! I'll try to keep you updated.
no apologies for my english, it's not my native language and I'm a slightly drunk at the moment ;-) ..
I just registered because I was reading this post about the TACTIII study here. You guys seem pretty curious and positive about it. I wasn't sure wheter to participate or not. Anyway I did it...
I'm from Germany and I have tinnitus since october 2013. It was going better until I reached
a level that I could live with. Then one weekend in January I went out to party (with propper earplugs of course) and ended up with a really horrible worsening. I decided to participate in the Am101 study. The worsening went away and I wasn't sure wheter I
should participate or not. I did because it semms pretty safe to do so. The doctor had done the AM101 studies before and she is also pretty porisitve about it. The drug Am101 is Ketamine, which is used and pretty safe. The method of application is: horrible but said to be pretty safe. First they numb your eardrum and then suck the numbing-liquid back out with a aspirator, that makes a horrible loud noise. Then you got a hole poked in it, which makes a horrible sound and is quite painfull. Then you get your inner ear filled with a liquid, that stays there for a coulple of days. On the second shot my eardrum was pretty sensitive and the injection was really painfull altough it got propper numbed. The doctor said, she thinks I got the real drug, because she thinks the drug made my eardrum more sensitive to pain and a placebo would not have done that. Besides she got a proband with the same issues before and he got the real drug. She needed to poke a new hole in my eardrum everyday, because it closed everytime. .. Ahmm.. decided to get it done just on one ear, although I got tinnitus on both ears. For people like us, with pretty 'sensitive' ears, that try to take care of them as much as possible, this procedure a real horror and pretty scarring. absolutly nothing for people with hyperacusis!
I got the three shots on monday, tuesday and wednesday. My right ear is still filled with that stuff, which I don't know if it's placebo or not. My tinnitus is a lot worse and my hearing changed because of the liquid that is still in my ear. I hope the liquid will run through the eustachian tube soon and leave me tinnitus free!!
This is my story with TACTIII so far, if you got any questions, please ask! I'll try to keep you updated.
no apologies for my english, it's not my native language and I'm a slightly drunk at the moment ;-) ..

 Member
Member thats what it takes to get anywhere!
thats what it takes to get anywhere!