We still don't know if there actually is tinnitus without any real hearing loss.Can anyone tell me if Lenire is supposed to help those with hearing loss related tinnitus?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Lenire — Bimodal Stimulation Treatment by Neuromod
- Thread starter Tinnitus Talk
- Start date
More options
Who Replied?- Jul 17, 2019
- 87
- Tinnitus Since
- 2017
- Cause of Tinnitus
- Idiopathic/Bad shoes?
Due respect Dave, when I said "vague" this was my only point. If we're going to measure the outcome of a treatment where the symptoms themselves are invisible to the observer we have only so many objective measurement criteria to use: THI and MML as I mentioned. Maybe you misunderstood me because nothing of what you wrote actually disagrees with what I was saying?To JayBowson:
It is, granted, not physical like a tumor but very akin to real pain and hence subjectively measurable.
If I'm reading this right you're hopeful for Shore's device but skeptical of Lenire? Why is this? Shore's device works off the exact same principle as Lenire. Neuromod has one of the foremost tinnitus experts in the world (Hubert Lim) working closely with them and Lenire has literally an order of magnitude more test subjects. Why favour the former and not the latter?When Lenire or a similar device is available, As the WHO said "I won't get fooled again."
I have been educated in recognizing the difference between a placebo and the real deal (and in fact I was very gratified when my ENT Doctor said that Dr. Shore's device does show real potential).
You're absolutely entitled to an opinion and I'm glad you shared it. My advice would be to seek an appointment with them and direct these questions at Caroline, their head consultant. She was across the trials and has been involved in consultations since they went to market.I have to disagree. Mild enough results fall within the gray-zone of placebo. They're also why all the other scam treatments persist. That kind of "does this or that snake-oil help?" word-of-mouth comprises 99% of the discussion of this forum. Go visit threads talking about Ring Ease or various herbals. A vague perception of an improvement is not enough proof of efficacy.
Based on the numbers in the charts that were in that fateful Vimeo video that was taken down, the best results people were getting (after they reprogrammed the timings in mid-stream) should translate to much more definitive testimonials than anything we've experienced so far outside of Clare B. So I don't think it's unreasonable for me to conclude that results so far have been "underwhelming".
I know people want to be optimistic. I do too, and I'm not trying to be a killjoy. But I can't get excited about what people are reporting. Not yet at least.
I understand getting an appointment with a clinic you have doubts about is somewhat counter-intuitive but to get answers to your questions from the highest authority on this subject, isn't.
As for the testimonials and feedback you're seeing... if somebody said to me, describe your tinnitus with a positive attitude, without sounding like a bag of spanners being jammed into a radio, I think I'd struggle too!
 Easier said than done.
Easier said than done. I'm interested as to what kind of information you want to see from the users? What would categorically sway you away from being a skeptic and believing that the treatment does work?
Unfortunately not, I have mid-range hearing loss and don't know when or how it occurred or whether it was hereditary or if that's even contributing to my tinnitus.Do you know what has made your tinnitus worse?
I generally got different answers depending on who I went to see and realised it really wasn't worth chasing.
Sorry I can't give you a conclusive answer on that one!
What about those with blood flow issues, TMJ, etc?We still don't know if there actually is tinnitus without any real hearing loss.
- Jul 17, 2019
- 87
- Tinnitus Since
- 2017
- Cause of Tinnitus
- Idiopathic/Bad shoes?
Peer review is just that. Reviews done by your peers. As such Neuromod is not in control of this process, and rightly so. These things take time. Sections to be rewritten, references updated, graphs to re-render etc etc... and scientific peer reviewers usually aren't paid for their work either, beyond a mention in the article so there's little incentive for them to work fast.OK, so about those numbers, what about the peer review? I am not trying to FUD Neuromod to death here but I have to admit being disappointed that we're this far downstream and not seeing more dramatic stories. The most dramatic testimony has been Allan's condition getting worse. It's hard to stay as upbeat as I once was given this situation.
Honestly I take a glass half full approach to the testimony. You allude to the general negative tone here all the time. These kinds of forums are outlets for people who suffer the most. Given that: Aren't you surprised that Lenire hasn't been ripped to shreds already? Like properly eviscerated? Like LEVO and Desyncra levels of destruction?

Instead what we have is a group of people who report improvement, a few more who report nothing much and @Allan1967 who has worsened. It's somewhat inconclusive whether his worsening was caused by noise exposure if I recall. All I'll say is reserve judgement and I sincerely hope he improves.
It might not. I'd honestly try to put those outliers out of your mind. Chances are massive you won't be one of them.Maybe I can find somewhere else that can do it as a walk-in, but I was stalling for the longest time in the hopes of hearing at least one more Clare B-grade positive testimonial but at this rate it doesn't look like it's gonna happen.
The way I figure it is this. On any given day I float between 25-40 THI. If I land smack bang in the middle (20 THI improvement), that's a 60% improvement (whatever that means in reality).
So is a 70% chance of a 60% improvement worth 2 thousand smackers? For me... probably? Depending on your baseline it may not be for you, only you can decide.
- Feb 11, 2019
- 801
- Tinnitus Since
- 01/2014
- Cause of Tinnitus
- Headcold/Flu
To Jay Bowson:
You are entirely correct about the subject's report of the effects of the treatment being the only measurable criterion for success.
Also, I know several people who have had this chronically for years who do not feel the need to take any therapeutic measures.
I find that this attains the very definition of incomprehensibly unbelievable.
This discrepancy reminds me of a truly instructive story regarding everyone's amazing variations in reactivity to nearly all phenomena.
An older gentlemen from the World War ll generation said that in the Service he saw super macho guys (like Burt Lancaster in "From Here to Eternity") who were fearless in combat, and the quintessence of toughness.
But when it came time to line up for inoculations, they would pass out at the sight of a needle.
This reminds me of the huge variation in pain/fear thresholds.
I must not allow my cynicism regarding the failure of everything else I have tried to prejudice me regarding this new treatment.
Since Dr. Shore is not far from Chicago, I am anticipating that for me her device will be the one that will be available soonest.
Although unsubstantiated, comments have been made that her treatment is applied to more nerve areas than Lenire's.
In January 2018 the Chicago Tribune had a major article about her research and projected applications.
It will be nearly two years since then, and I sincerely hope that this is available in 2020.
I have to also remember that no medical procedure is ever guaranteed.
You are entirely correct about the subject's report of the effects of the treatment being the only measurable criterion for success.
Also, I know several people who have had this chronically for years who do not feel the need to take any therapeutic measures.
I find that this attains the very definition of incomprehensibly unbelievable.
This discrepancy reminds me of a truly instructive story regarding everyone's amazing variations in reactivity to nearly all phenomena.
An older gentlemen from the World War ll generation said that in the Service he saw super macho guys (like Burt Lancaster in "From Here to Eternity") who were fearless in combat, and the quintessence of toughness.
But when it came time to line up for inoculations, they would pass out at the sight of a needle.
This reminds me of the huge variation in pain/fear thresholds.
I must not allow my cynicism regarding the failure of everything else I have tried to prejudice me regarding this new treatment.
Since Dr. Shore is not far from Chicago, I am anticipating that for me her device will be the one that will be available soonest.
Although unsubstantiated, comments have been made that her treatment is applied to more nerve areas than Lenire's.
In January 2018 the Chicago Tribune had a major article about her research and projected applications.
It will be nearly two years since then, and I sincerely hope that this is available in 2020.
I have to also remember that no medical procedure is ever guaranteed.
I've been following this thread closely and trying to digest the information in order to determine where my pessimism/optimism barometer should point.I'm interested as to what kind of information you want to see from the users? What would categorically sway you away from being a skeptic and believing that the treatment does work?
A big reason I'm skeptical right now is that I was under the assumption that the majority of the improvement should be expected within the first six weeks. This is exactly why they experimented with changing up the timings. The thinking goes that your brain is somehow building up a tolerance to the treatment similar to how you should change up a workout regimen. That's what Hubert Lim was talking about in that video. So now that we're over the six week mark and nobody's even reporting as much improvement as the best responders in the PowerPoint chart.
Of course, later on there was that hearsay where Neuromod told someone that the 12 week treatment schedule was somewhat arbitrary and that they can't rule out further improvement with (quote unquote) "continued use". @kelpiemsp's results with the other device had him using it for longer than Lenire, so it may very well be that treatment doesn't plateau but makes inroads gradually or in stair-step fashion.
These two schools of thought seem mutually exclusive, though. I would think that if someone continued to use this device religiously for an extended period of time, like 6+ months, already burned through a tongue-tip and is on a replacement, then it's fair to say it's a bust, just as much as I finally gave up bioflavonoids after using it for over 90 days.
Sure, people here are still a small sample size so it's hard to draw a conclusion, but all I'd really want to see is one, at least one, high-profile member say that it's making a genuine unambiguous improvement. We haven't seen that yet based on the verbiage.
So I've gone from optimistic to slightly pessimistic. If you fast-forward another six weeks when their treatment officially ends and they enter the "continued use" phase and the same people still can't say their tinnitus has improved, then I'll be far more pessimistic. It's not that I think they are consciously scamming, but I just have greater trust of the personal testimonials via this forum than I do the charts and graphs from Neuromod. So if the results in the field don't measure up to Neuromod's hype, I'm going to judge this device's efficacy based on these independent forum testimonials.
Of course, by then I will have long decided whether to follow through or not so I may just be one of the testimonials. We shall see.
2022 to 2024 if we are lucky. Let's be realistic.It will be nearly two years since then, and I sincerely hope that this is available in 2020.
Perhaps he likes Budweiser and not Guinness.Due respect Dave, when I said "vague" this was my only point. If we're going to measure the outcome of a treatment where the symptoms themselves are invisible to the observer we have only so many objective measurement criteria to use: THI and MML as I mentioned. Maybe you misunderstood me because nothing of what you wrote actually disagrees with what I was saying?
If I'm reading this right you're hopeful for Shore's device but skeptical of Lenire? Why is this? Shore's device works off the exact same principle as Lenire. Neuromod has one of the foremost tinnitus experts in the world (Hubert Lim) working closely with them and Lenire has literally an order of magnitude more test subjects. Why favour the former and not the latter?
It won't be Tinnituszentrum in Regensburg - AFAIK. But it is just rumours.Do you have any information where in Germany do they plan to expand it?
I know someone who is planned for a Lenire treatment in October in Germany.
Since it is all very vague yet, I don't want to give more information.
But as far as I know more, I will let you know. Please don't ask via PM. I will not share more.
It is just that we hopefully see availability (even not in masses) this year in Germany and probably more next year.
- Jul 8, 2019
- 1,185
- Tinnitus Since
- 1991
- Cause of Tinnitus
- Loud Music / family history
Me neither. But I personally don't feel at this point there's enough literature in any one single place to make an informed decision about the efficacy of Lenire. I do feel Neuromod may have jumped the gun a little by launching now. The alternative is to wait for the Shore device. But if you read what @linearb said, Susan Shore informed him personally that her device may not be on the market until 2024.I am neither optimistic nor pessimistic
I don't speak for anyone but myself, but as I mentioned before, choosing between a device that I feel is soft-launched and still in development, or waiting circa 5 years for an alternate device release is the rock and hard place I find myself in. It's got nothing to do with "investigative negative theories" (whatever they are), or optimism/pessimism for that matter. It's simply an adjustment of expectations based on the landscape as I see it.
Fabrikat
Member
- May 1, 2017
- 416
- Tinnitus Since
- 1973
- Cause of Tinnitus
- Otosclerosis then volume then viral infection
Don't hold your breath. Susan Shore is an excellent research scientist, but sadly she's in no rush whatsoever to get her device to market.I sincerely hope that this is available in 2020.
Oh, I was hoping for Regensburg, it's nearer to my home... somewhere in north is almost as far as Dublin for me.It won't be Tinnituszentrum in Regensburg - AFAIK.
Please, share information when you feel you can. It will be useful for all of us waiting for some nearer destination.
I don't understand why Regensburg wasn't chosen for the German launch?! Perhaps they don't have the business organization for such new therapy, since they are a clinic.
There is also the Charité clinic in Berlin, but they focus mostly on CBT.
So it must be an ENT I expect.
Was the therapy duration for Lenire extended from 12 to 18 weeks or not? I remember somebody here was posting this.
There is also the Charité clinic in Berlin, but they focus mostly on CBT.
So it must be an ENT I expect.
Was the therapy duration for Lenire extended from 12 to 18 weeks or not? I remember somebody here was posting this.
That's my cue.Don't hold your breath. Susan Shore is an excellent research scientist, but sadly she's in no rush whatsoever to get her device to market.
I think last time I made my obligatory GIF post someone cried foul and said Shore herself is a sufferer and therefore a stakeholder (as in, physician, heal thyself), to which I wonder, has she used her OWN device and if so why doesn't she relate her own results?
- Feb 17, 2017
- 10,400
- Tinnitus Since
- February, 2017
- Cause of Tinnitus
- Acoustic Trauma
The chart reports the improvement after 12 weeks of treatment. Hopefully the people with severe tinnitus will be able to continue to experience improvement. In order to get their THI below 10, they might just need to have more treatments done.This tells me a few things.
1. If you have severe-catastrophic tinnitus, you can still see an excellent reduction in handicap, but it won't be as dramatic as those below a THI of 70.
2. If you're below a THI of 70, it's possible for your tinnitus to fade entirely or become a non-factor.
3. Those below a 60 had the biggest reduction, with a 50 and 30 at entry reporting a 0 at exit, and most were below 30.
The number 3 is especially telling. It seems like people with moderate tinnitus are really given a huge push by this device, like anything below, say, a 40 on the scale is not too difficult to deal with.
Have you even emailed her? She has said 2020 is her goal just a few months ago in someone's email, and also said that in an interview with the ATA.Don't hold your breath. Susan Shore is an excellent research scientist, but sadly she's in no rush whatsoever to get her device to market.
Thank you Edward. I find the answer straightforward and professional (although most probably it wasn't written by Dr. Shore herselfThis is an email I received from Dr. Shore on August 30, 2019.
I sent her an email asking when she was expecting her device to be out in the market. I also told her about Lenire and how it was looking promising. This is what she replied to me.
View attachment 32006
 )
)Do we know if there is a way to get added on this e-mail list of Dr. Shore's?
I sent her an email at sushore@umich.edu and asked her if I lived in Miami if I could be a part of the trials and she told me she'd add me to her mailing list for updates.Thank you Edward. I find the answer straightforward and professional (although most probably it wasn't written by Dr. Shore herself)
Do we know if there is a way to get added on this e-mail list of Dr. Shore's?
- Sep 17, 2017
- 234
- Tinnitus Since
- 2017
- Cause of Tinnitus
- Medical mistake
I emailed her today asking about the time to market.This is an email I received from Dr. Shore on August 30, 2019.
I sent her an email asking when she was expecting her device to be out in the market. I also told her about Lenire and how it was looking promising. This is what she replied to me.
View attachment 32006
She responded: "Unfortunately bringing to market is complicated - we first will go for FDA approval after the trial."
2020: Trial Finishes.
How long does FDA approval take?
What happens after FDA approval, why does she say "it's complicated to bring to market".
2022 to 2024 if it ever comes to market.I emailed her today asking about the time to market.
She responded: "Unfortunately bringing to market is complicated - we first will go for FDA approval after the trial."
2020: Trial Finishes.
How long does FDA approval take?
What happens after FDA approval, why does she say "it's complicated to bring to market".
These things take time...
Susan is a PhD, an acclaimed and successful doctor. They don't spend their free time making claims or getting people overexcited. I grew up with scientists, they arr a special breed, they would never behave like us on this forum... We would make a great army given adequate hearing protection.That's my cue.
View attachment 31998
I think last time I made my obligatory GIF post someone cried foul and said Shore herself is a sufferer and therefore a stakeholder (as in, physician, heal thyself), to which I wonder, has she used her OWN device and if so why doesn't she relate her own results?
G
GoatSheep
Guest
2022 to 2024 if it ever comes to market.
These things take time...
Fabrikat
Member
- May 1, 2017
- 416
- Tinnitus Since
- 1973
- Cause of Tinnitus
- Otosclerosis then volume then viral infection
No one would be happier than me to see this come out by 2020. I'll be standing right behind you in the line to get into the clinic.Have you even emailed her? She has said 2020 is her goal just a few months ago in someone's email, and also said that in an interview with the ATA.
Susan Shore has been working on this for nigh on twenty years. People on Tinnitus Talk have participated in her earlier trials with successful results, years ago. Tinnitus Talk members have been reporting her achievements since 2013. No one can doubt her commitment to proper scientific process.
For whatever reason, she has taken her sweet time pushing this forward and had earlier suggested 2022 to 2024 as her expected timeline. I may be wrong, but I've only heard her speak of a commercial partner in the last 12 months.
But to bring this back to Lenire, whilst Susan Shore walks to the beat of her own drum, maybe Lenire's introduction onto the scene has inspired the will to bring the date forward to 2020. Who knows? I hope so.
Knowing how long these things take, if the trial ends next year and then we have to wait for the FDA to do its work, I somehow can't help thinking it's going to take longer than 2020.
Can anyone tell me if Lenire is supposed to help those with hearing loss related tinnitus?
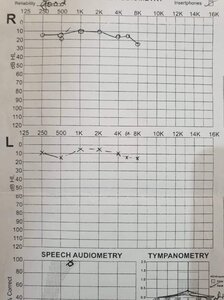
JohnAdams, I would tend to agree with you, below is my hearing test and my audiologist said that I had minimal hearing loss, I still have some hearing loss mostly in my right ear where my tinnitus is worse and fluctuates the most, from pounding to hardly audible!We still don't know if there actually is tinnitus without any real hearing loss.
If their idea of tinnitus with no hearing loss is someone like me, then that's a false comparison, I'd assume they don't really know what no hearing loss looks like especially as they usually only test within the speech range of 8000 Hz so without scientifically testing it by taking someone of a young age with no hearing loss and monitoring them for the next 50 odd years of their life protecting the heck out of their ears.
Don't think they'd get many volunteers to do that.
Because Susan Shore has invested far more time in rigorous scientific studies. That's a big difference for a start.Any idea why Susan Shore said her treatment is not the same as Lenire?
Also is her product still expected to be in the market in 2020?
I wrote to the esteemed Stephen Heller of Stanford University, the Godfather of hearing regeneration and he responded to me personally. These people are human with hearts.Thank you Edward. I find the answer straightforward and professional (although most probably it wasn't written by Dr. Shore herself)
Do we know if there is a way to get added on this e-mail list of Dr. Shore's?

 Member
Member